India’s rising cancer burden is creating an urgent—and lucrative—demand for specialized oncology centers. What was once seen purely as a healthcare necessity is now a high-growth business opportunity. Leading hospital chains report that over 25% of their revenue now comes from oncology services alone.
For medical entrepreneurs, investors, and clinicians, this presents a powerful opening. But make no mistake—figuring out how to start an oncology center in India is no easy task. From navigating complex licensing laws to securing multi-crore funding and hiring top-tier talent, the path is anything but simple.
This guide breaks it all down. Whether you’re building from the ground up or expanding an existing facility, you’ll find a step-by-step blueprint tailored to India’s unique healthcare landscape—from business planning and regulatory hurdles to staffing, equipment, and operational success.

Table of Contents
The Opportunity: Why Invest in an Oncology Center in India?
The decision to start an oncology center in India is driven by rising cancer cases, strong market demand, and government support. With limited cancer care infrastructure and growing economic potential, the sector offers a major opportunity for private investment.
Market Dynamics: A Growing Need
The Indian healthcare market, valued at approximately $180 billion in FY 2023, is projected to expand at a compound annual growth rate (CAGR) of around 12% to reach $320 billion by 2027 . Within this booming sector, oncology stands out as a critical and rapidly growing vertical.
- Rising Cancer Incidence: India is facing a sharp rise in cancer cases. Late diagnosis and limited access to treatment often cause preventable deaths. This surge in patients highlights the urgent need to start oncology centers and expand specialized treatment facilities.
- Infrastructure Gap: India has only 0.5 hospital beds per 1,000 people, far below the WHO’s 3–5 bed standard. The shortage is worse in specialized care like radiation oncology, where most centers run at full capacity, leading to long treatment delays. Expanding facilities and starting new oncology centers is essential to bridge this gap.
- Dominance of Private Sector: The private sector is the backbone of Indian healthcare, accounting for about 74% of the country’;s total healthcare infrastructure . This reliance creates a clear pathway for private investment to fill the existing gaps.
- High Revenue Potential: Oncology has become a major profit driver for hospitals in India. In Q1FY26, it contributed over 25% of revenue at Max Healthcare, while Fortis reported a 28% YoY rise, with oncology now forming 16% of its income. HCG, India’s largest cancer network, achieved 19% CAGR growth from FY20–FY24. These trends show why many investors aim to start oncology centers as a high-return opportunity.
Government Initiatives & Support
Government Support: While the private sector leads, the government is creating a supportive environment for cancer care. Programs like NP-NCD fund State Cancer Institutes (SCIs) and Tertiary Cancer Care Centres (TCCCs), signaling cancer as a national priority. Meanwhile, Make in India and PLI schemes for medical devices reduce equipment costs, making it easier and more affordable for investors to explore how to start an oncology center in India.
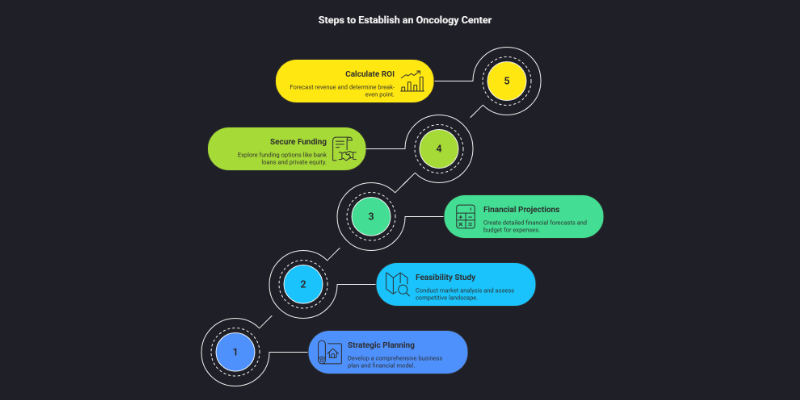
Step 1: Strategic Business Planning & Financial Modeling for the Indian Market
A meticulously crafted business plan is the cornerstone of success when exploring how to start an oncology center. It converts vision into a viable, fundable project and acts as a critical risk-mitigation tool in India’s complex healthcare market. Skipping or rushing this stage is one of the most costly mistakes, often leading to financial distress and operational failure.
Phase I: Feasibility Study and Market Analysis
Before a single rupee is spent, a comprehensive feasibility study is non-negotiable. This initial step provides the data-driven justification for the project and shapes its strategic direction .
- Market Needs Assessment: The first task is to understand the demand. This involves a deep dive into the proposed service area.
- Demographics & Epidemiology: Analyze the population density, age distribution, and income levels of the target catchment area. Crucially, research the prevalence of specific cancer types common to the region. This data will inform your service mix and equipment choices.
- Location Analysis (Tier-1 vs. Tier-2/3): The dynamics differ vastly. A Tier-1 city like Mumbai or Bengaluru offers higher patient affordability but faces intense competition and staggering real estate costs. A Tier-2 or Tier-3 city may present a larger unmet need and lower operational costs but could face challenges in attracting specialized talent and may have lower per-patient revenue potential.
- Demographics & Epidemiology: Analyze the population density, age distribution, and income levels of the target catchment area. Crucially, research the prevalence of specific cancer types common to the region. This data will inform your service mix and equipment choices.
- Competitive Landscape: A thorough analysis of existing players is vital to carve out a niche.
- Map all competitors within a defined radius (e.g., 10-20 km), including large multi-specialty hospital chains (like Apollo, Fortis), other standalone cancer centers, and government facilities.
- Analyze their service offerings (Do they have radiation oncology? Robotic surgery?), pricing structures, patient volumes, and perceived quality. This helps identify service gaps your center can fill.
- Map all competitors within a defined radius (e.g., 10-20 km), including large multi-specialty hospital chains (like Apollo, Fortis), other standalone cancer centers, and government facilities.
- Service Mix & Business Model: Based on the market analysis, define the scope of your center.
- Comprehensive Center: Offering the full spectrum of care: Diagnostics (Imaging, Pathology), Medical Oncology (Chemotherapy, Immunotherapy), Radiation Oncology, Surgical Oncology, and Palliative Care. This model has the highest revenue potential but also the highest CapEx.
- Hub-and-Spoke Model: A central comprehensive center (hub) connected to smaller satellite clinics (spokes) in surrounding towns that offer basic diagnostics and day-care chemotherapy. This model, used by players like HCG, allows for wider reach and efficient resource use .
- Specialized Boutique Clinic: Focusing on a specific niche, such as a day-care chemotherapy and infusion center. This model has a significantly lower entry barrier and can be a good starting point.
- Comprehensive Center: Offering the full spectrum of care: Diagnostics (Imaging, Pathology), Medical Oncology (Chemotherapy, Immunotherapy), Radiation Oncology, Surgical Oncology, and Palliative Care. This model has the highest revenue potential but also the highest CapEx.
Phase II: Detailed Financial Projections (in INR)
Translating the strategic plan into a detailed financial model is the next crucial step. Investors and lenders will scrutinize these numbers to assess the project’s viability. The costs are substantial and vary significantly based on location and scale.
Capital Expenditure (CapEx) Breakdown
CapEx represents the initial, one-time investment required to build and equip the center. Equipment and construction are typically the largest components .
| Cost Item | Estimated Range (INR) for a 50-100 Bed Center | Key Considerations & Data Points |
|---|---|---|
| Land & Construction | ₹25 Cr – ₹100+ Cr | Cost per bed is a key metric: ₹50–90 lakh in Tier-1 cities vs. ₹30–45 lakh in Tier-2/3 cities . NABH standards for facility design and AERB requirements for radiation bunkers add to the cost. |
| Medical Equipment | ₹15 Cr – ₹60+ Cr | A single Linear Accelerator (LINAC) for radiation can cost ₹15-25 Cr. A PET-CT scanner costs ₹8-12 Cr, and an MRI ₹5-10 Cr. Costs can be influenced by “Make in India” options and import tariffs . |
| Licenses & Approvals | ₹50 Lakh – ₹2 Cr | This includes statutory fees and consultant charges for navigating the complex approval process involving AERB, NABH, State Pollution Control Board, Fire NOC, etc. . |
| Initial Staffing & Training | ₹3 Cr – ₹10+ Cr | Covers recruitment costs and salaries for the first 6-12 months. A senior Medical/Radiation Oncologist can command ₹30 Lakh to over ₹1 Crore annually . |
| Working Capital | ₹5 Cr – ₹15 Cr | This is a critical and often underfunded component. It covers all operational expenses for the initial 6-12 months before revenue streams stabilize and become predictable. |
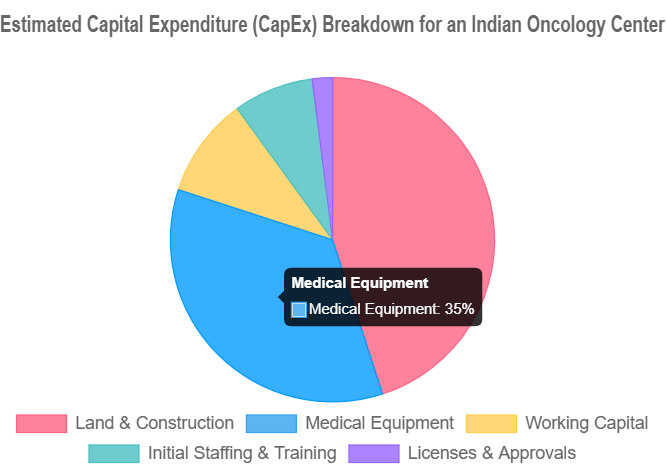
Note: The figures above are indicative for a mid-sized comprehensive center. A smaller, specialized day-care facility could have a significantly lower CapEx, potentially in the ₹2-5 Crore range, by avoiding the high costs of radiation and advanced imaging equipment.
Operational Expenditure (OpEx) Model
OpEx includes all the recurring costs required to run the center day-to-day. A sustainable model must accurately forecast these expenses.
- Staff Salaries: The largest component of OpEx. This includes clinical, nursing, technical, and administrative staff.
- Consumables & Pharmacy: Costs of drugs (especially high-cost chemotherapy and immunotherapy agents), and medical supplies.
- Equipment Maintenance: Annual/Comprehensive Maintenance Contracts (AMCs/CMCs) for high-end equipment are essential and can cost 5-8% of the equipment’s value annually. A government report suggests budgeting for a 2-year warranty followed by an 8-year CMC .
- Utilities: Electricity (especially for high-energy equipment like LINACs), water, and internet.
- Marketing & Business Development: Costs for awareness campaigns, doctor outreach programs, and digital marketing.
- Administrative Overheads: Rent (if applicable), insurance, security, housekeeping, and other administrative costs.
Phase III: Securing Funding in India
With a solid business plan and financial model, the next step is to secure funding. The Indian market offers several avenues:
- Bank Loans: Major Indian banks like SBI, HDFC, and ICICI have dedicated healthcare financing divisions. They typically offer term loans for capital expenditure and working capital loans. Be prepared for stringent due diligence and collateral requirements.
- Private Equity (PE) & Venture Capital (VC): The Indian healthcare sector is a major focus for PE and VC funds. Firms like Sequoia Capital India (now Peak XV Partners), Chiratae Ventures, and others actively invest in promising healthcare models. They look for a strong management team, a scalable business model, and a clear competitive advantage .
- Government Schemes: While direct funding for private centers is limited, some state governments offer incentives or support through health infrastructure funds. These are worth exploring, especially if the project aligns with public health goals.
- Public-Private Partnerships (PPPs): Partnering with government bodies can be a viable model, particularly for establishing facilities in underserved regions. This can involve the government providing land or infrastructure while the private partner manages operations .
Phase IV: Calculating ROI and Break-Even Point
Investors will want to see a clear path to profitability. This involves forecasting revenue and calculating key financial metrics.
- Revenue Forecasting: A simplified revenue model can be built using the formula:
Total Revenue = (Average daily patient volume × Average revenue per patient) + Ancillary revenue (Pharmacy, Diagnostics, etc.). Revenue per patient will vary greatly depending on the treatment modality (e.g., a radiation therapy course is much higher value than a single consultation). - Break-Even Analysis: This determines the point at which total revenue equals total costs. The formula is:
Break-Even Point (in patients) = Total Fixed Costs / (Average Revenue per Patient - Average Variable Cost per Patient). For a new hospital project in India, the break-even period is typically estimated to be between 5 to 8 years, depending on scale, location, and operational efficiency . - Return on Investment (ROI): This measures the profitability of the investment. The formula is:
ROI = (Net Annual Profit / Total Investment) × 100%. A target ROI will depend on the risk profile, but investors in the Indian healthcare sector often look for benchmarks that outperform standard market returns.
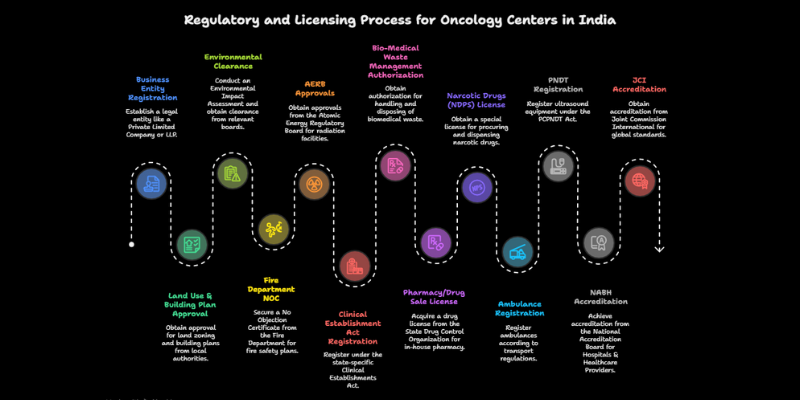
Step 2: Navigating India’s Complex Regulatory & Licensing Maze
Navigating licenses and approvals is the most daunting phase of how to start an oncology center in India. The process is not single-window; it requires sequential applications to central, state, and local authorities. Underestimating this complexity often leads to costly delays. A realistic approval timeline is 12–24 months.
The Essential Pre-Construction Approval Checklist
These approvals are mandatory before you can even break ground. The process is sequential, meaning one approval is often a prerequisite for the next.
[ ]Business Entity Registration: First, establish the legal entity. This is typically a Private Limited Company or a Limited Liability Partnership (LLP) to limit personal liability.[ ]Land Use &; Building Plan Approval: The land must be zoned for healthcare/commercial use. The building plans must be approved by the local Municipal Corporation or development authority. This is a major milestone .[ ]Environmental Clearance: For larger hospitals (often defined by bed capacity or built-up area), an Environmental Impact Assessment (EIA) study and subsequent clearance from the State Pollution Control Board (SPCB) or Ministry of Environment, Forest and Climate Change (MoEFCC) is mandatory. This can be a lengthy process.[ ]Fire Department NOC (No Objection Certificate): The fire safety plan, including evacuation routes, fire suppression systems, and alarms, must be approved by the local Fire Department. This NOC is essential for obtaining the building plan approval.[ ]AERB (Atomic Energy Regulatory Board) Approvals: This is the most critical and time-consuming approval for an oncology center with radiation facilities. It is a multi-stage process managed through the eLORA (e-Licensing of Radiation Applications) portal . The key stages include:
- Site Approval: Approval of the proposed location for the radiation facility.
- Layout & Shielding Design Approval: The architectural plans for the radiation bunkers must be approved by AERB to ensure adequate radiation shielding.
- Procurement Permission: Permission to purchase the radiation equipment (e.g., LINAC, Brachytherapy unit).
- Installation & Commissioning Approval: Approval to install the equipment and perform quality assurance tests.
- License for Operation: The final license to start treating patients, issued after successful inspection and verification of all safety protocols and qualified personnel.
- Site Approval: Approval of the proposed location for the radiation facility.
The Mandatory Operational Licensing Checklist
Once construction is complete and equipment is installed, a new set of licenses is required before the center can legally begin operations.
[ ]Clinical Establishment Act Registration: Registration under the state-specific Clinical Establishments (Registration and Regulation) Act is mandatory. This act sets minimum standards for infrastructure and human resources.[ ]Bio-Medical Waste Management Authorization: An authorization from the State Pollution Control Board (SPCB) is required for the safe handling, treatment, and disposal of biomedical waste, as per the Bio-Medical Waste Management Rules.[ ]Pharmacy/Drug Sale License: If the center has an in-house pharmacy, a drug license from the State Drug Control Organization is required.[ ]Narcotic Drugs (NDPS) License: A special license is needed to procure, store, and dispense narcotic drugs like morphine, which are essential for cancer pain management.[ ]Ambulance Registration: The center’s ambulances must be registered as such and equipped according to transport regulations.[ ]PNDT Registration: If the center uses ultrasound equipment, registration under the Pre-Conception and Pre-Natal Diagnostic Techniques (PCPNDT) Act is required.
Achieving Quality Standards: NABH & JCI Accreditation
While not always legally mandatory to open, quality accreditation is practically essential for long-term success and financial viability in India.
- Why Accreditation Matters: For anyone exploring how to start an oncology center, accreditation from NABH or JCI is vital. It builds patient trust and is essential for empanelment with insurance companies, TPAs, and government health schemes like CGHS and ECHS. Without accreditation, centers lose access to insured patients, severely limiting revenue potential.
- The NABH Process (National Accreditation Board for Hospitals & Healthcare Providers): NABH is India’s premier accreditation body, and its standards are tailored to the Indian context while aligning with global best practices . The process is rigorous and involves:
- Preparation: The hospital must procure the NABH standards book (which contains over 600 objective elements for hospitals), conduct an internal gap analysis to identify areas of non-compliance, and implement corrective actions.
- Application: An online application is submitted through the NABH portal.
- Pre-Assessment: NABH assessors conduct an initial review to check the hospital’s readiness for the final survey.
- Final Assessment: A multi-day, on-site survey where a team of assessors evaluates the hospital’s compliance with every standard, covering patient care, management, and infrastructure.
- Accreditation: If the hospital meets the standards, it is granted accreditation, which is typically valid for three years, after which a reassessment is required.
- Preparation: The hospital must procure the NABH standards book (which contains over 600 objective elements for hospitals), conduct an internal gap analysis to identify areas of non-compliance, and implement corrective actions.
- JCI Accreditation (Joint Commission International): JCI is the global gold standard in healthcare accreditation. For those researching how to start an oncology center, JCI is a powerful differentiator—helping attract medical tourists and positioning the facility as an international center of excellence. Several leading cancer hospitals in India hold JCI status, proving their commitment to world-class quality and patient safety. The process mirrors NABH but is assessed against global standards by international surveyors.
Step 3: Designing the Facility, Procuring Equipment & Building Your Team
With the business plan and regulatory roadmap in place, the focus shifts to the physical and human infrastructure. This phase involves translating the vision into a tangible, functional, and high-quality care environment.
Evidence-Based Facility Design & Layout
The physical design of a cancer center profoundly impacts patient experience, operational efficiency, and clinical outcomes. The goal is to create a space that is not only clinically effective but also emotionally supportive .
- Patient-Centric Principles:
- Patient Flow Optimization: The layout should be logical and intuitive to minimize patient movement and reduce stress. This means co-locating complementary services: consultation rooms near examination areas, the pharmacy near the chemotherapy bay, and changing rooms near radiation therapy units .
- Creating a Healing Environment: When planning how to start an oncology center, design plays a vital role in patient care. Cancer treatment is emotionally and physically exhausting, so facilities should promote calm and comfort. Key elements include natural light, soothing colors, nature-inspired interiors, clear wayfinding, and private rooms for sensitive consultations.
- Patient Flow Optimization: The layout should be logical and intuitive to minimize patient movement and reduce stress. This means co-locating complementary services: consultation rooms near examination areas, the pharmacy near the chemotherapy bay, and changing rooms near radiation therapy units .
- Clinical Area Design (Indian Context):
- Radiation Bunkers: A critical step in how to start an oncology center is designing radiation bunkers that meet strict AERB guidelines. These rooms require specific concrete wall thickness, a maze-style entrance to prevent radiation scatter, and minimum dimensions (such as 3-meter height) to safely house equipment and patient transfers.
- Chemotherapy Infusion Bay: In planning how to start an oncology center, chemotherapy bay design is crucial for safety and comfort. Nursing staff must have clear line-of-sight to all patients, often achieved with a central nursing station. For a better patient experience, large areas can be divided into smaller clusters of 6–10 chairs with easy access to refreshments.
- Infection Control: A key aspect of how to start an oncology center is ensuring strict infection control. Cancer patients are highly vulnerable, so facilities must include NABH-compliant isolation rooms—standard pressure for infectious cases and negative pressure rooms for patients with airborne diseases.
- Radiation Bunkers: A critical step in how to start an oncology center is designing radiation bunkers that meet strict AERB guidelines. These rooms require specific concrete wall thickness, a maze-style entrance to prevent radiation scatter, and minimum dimensions (such as 3-meter height) to safely house equipment and patient transfers.
Essential Equipment Procurement Strategy
The technology and equipment are the heart of an oncology center, enabling accurate diagnosis and effective treatment. This is a major capital expense that requires careful planning.
Core Equipment List
| Category | Key Equipment | Notes for the Indian Market |
|---|---|---|
| Radiation Oncology | Linear Accelerator (LINAC), CT Simulator, 3D Treatment Planning System (TPS), High-Dose-Rate (HDR) Brachytherapy Unit | All radiation-emitting equipment must have type-approval from the AERB before it can be imported or installed. This is a critical checkpoint in the procurement process . |
| Medical Imaging | PET-CT Scanner, 3T MRI, Digital Mammography with Tomosynthesis, High-resolution Ultrasound, Digital X-ray | Advanced diagnostics are not only crucial for accurate staging and treatment planning but also serve as significant revenue streams for the center. |
| Surgical Oncology | Modular Operation Theatres with Laminar Flow, Advanced Laparoscopic Systems, Robotic Surgical Systems (e.g., da Vinci), Anesthesia Workstations | Robotic surgery is a growing trend and a major differentiator, especially in metro and Tier-1 city centers, attracting both patients and top surgical talent. |
| Laboratory & Pathology | Histopathology Lab (for tissue diagnosis), Immunohistochemistry (IHC) Analyzers, Tumor Marker Analyzers, Frozen Section capability | An in-house, high-quality pathology department is crucial for quick turnaround times, which is essential for efficient Multidisciplinary Team (MDT) meetings and treatment planning. |
Procurement Tips for India
- Budget for Lifecycle Costs: The initial purchase price is only part of the cost. Always factor in the price of multi-year Annual/Comprehensive Maintenance Contracts (AMCs/CMCs), which are vital for ensuring equipment uptime. Budgeting for an 8-10 year service contract post-warranty is a standard practice.
- Evaluate “Make in India” Options: The Indian government is actively promoting domestic manufacturing of medical devices. While the most advanced technologies may still be imported, evaluating locally manufactured equipment can potentially reduce costs, mitigate currency fluctuation risks, and ensure better and faster service support.
- Regulatory Compliance: Ensure that any equipment you purchase, especially imported items, complies with all Indian regulations, including those from the Central Drugs Standard Control Organization (CDSCO) and AERB.
Building Your Core Multidisciplinary Team (MDT)
A building and its equipment are useless without the right people. High-quality cancer care is delivered by a coordinated Multidisciplinary Team (MDT), where specialists from different fields collaborate to create an optimal treatment plan for each patient. This approach is considered the standard of care and is proven to improve patient outcomes .

- Core Team Composition:
- Clinical Leads: Medical Oncologist, Radiation Oncologist, and Surgical Oncologist(s). These are the pillars of the treatment team.
- Diagnostic Leads: Radiologist (specializing in onco-imaging) and Pathologist (specializing in onco-pathology). Their expertise is fundamental to accurate diagnosis and staging.
- Essential Technical & Support Staff:
- Medical Physicist & Radiation Safety Officer (RSO): These roles are mandatory AERB requirements for any facility with radiation equipment. They are responsible for quality assurance, treatment planning verification, and radiation safety.
- Oncology Nurses: Specialized nurses trained in chemotherapy administration, patient care, and symptom management. Certification from bodies like the Oncology Nursing Society (ONS) is a significant value-add.
- Pharmacists: Preferably with experience in oncology pharmacy, responsible for safe preparation and dispensing of chemotherapy drugs.
- Support Professionals: Counselors, nutritionists, palliative care specialists, and social workers are integral to providing holistic, patient-centric care.
- Medical Physicist & Radiation Safety Officer (RSO): These roles are mandatory AERB requirements for any facility with radiation equipment. They are responsible for quality assurance, treatment planning verification, and radiation safety.
- Clinical Leads: Medical Oncologist, Radiation Oncologist, and Surgical Oncologist(s). These are the pillars of the treatment team.
- Staffing Norms & Recruitment in India:
- Recruitment Challenges: There is a significant shortage of trained oncologists and specialized healthcare professionals in India. The oncologist-to-patient ratio is estimated to be as low as 1:2000 . Recruiting and retaining top talent, especially in Tier-2 and Tier-3 cities, is a major challenge.
- Staffing Ratios: While there isn’t a single mandated national standard, various committees and regulatory bodies provide recommendations for nurse-to-patient ratios. These vary for inpatient wards, ICUs, and outpatient/ambulatory settings . Your staffing plan should be based on these guidelines and the specific acuity of your patient population.
- Retention Strategy: To retain valuable staff, centers must offer competitive salaries, a positive and supportive work environment, opportunities for continuous professional development, and a culture that values their contribution to patient care.
- Recruitment Challenges: There is a significant shortage of trained oncologists and specialized healthcare professionals in India. The oncologist-to-patient ratio is estimated to be as low as 1:2000 . Recruiting and retaining top talent, especially in Tier-2 and Tier-3 cities, is a major challenge.
Common Mistakes to Avoid When Building a Hospital in India
The path to building a healthcare facility in India is littered with potential pitfalls. Learning from the common mistakes of others can save invaluable time, money, and frustration. Based on insights from industry experts and project management consultants, here are the critical errors to avoid Actiss Healthcare, 2025; .
Planning & Design Errors
- Inadequate Site Analysis: Choosing a location based solely on cost without a deep analysis of accessibility for ambulances and patients, local competition, catchment area demographics, and future infrastructure development plans in the area.
- Ignoring Local Climate Considerations: Using a one-size-fits-all building design across different climate zones in India. A design for arid Rajasthan will fail in humid Kerala if ventilation, drainage, and material selection are not adapted.
- Not Planning for Future Expansion: Designing a facility that is landlocked and has insufficient space for future growth. Underestimating the space needed for parking (a major issue in India), new equipment, or additional bed capacity is a common long-term strategic failure.
Regulatory & Legal Oversights
- Underestimating Regulatory Timelines: The single biggest mistake is assuming approvals will be quick. The multi-stage processes for AERB and Environmental Clearances can take 12-24 months alone. A realistic project timeline must be built around these regulatory milestones, not the other way around.
- Ignoring State-Specific Regulations: Assuming central government guidelines are sufficient. Many rules, such as those for bio-medical waste management, staff accommodation, and building codes, vary significantly from state to state. What is compliant in Maharashtra may not be in Tamil Nadu.
- Poor Documentation: Failing to maintain a meticulous, centralized record of all applications, approvals, NOCs, and correspondence with regulatory bodies. This leads to massive delays during inspections and renewals.
Financial Miscalculations
- Underfunding Working Capital: This is a fatal error. Many entrepreneurs focus heavily on CapEx but fail to budget enough cash to cover operational expenses (salaries, utilities, supplies) for the first 12-18 months, a period where revenue is low and unpredictable.
- Ignoring Hidden Lifecycle Costs: The business plan must account for costs beyond the initial purchase. This includes multi-year AMCs/CMCs for equipment, software subscription and upgrade fees, accreditation renewal costs, and periodic technology replacement.
- Unrealistic Revenue Projections: Overly optimistic projections about patient volumes and reimbursement rates can lead to a flawed business plan and a failure to secure funding or meet loan obligations.
Operational Pitfalls
- Compromising on Key Hires: In a rush to open, some founders compromise on the quality and experience of their core team, especially the Chief Medical Officer, Head of Nursing, and key oncologists. This directly impacts clinical quality and reputation.
- Delaying Marketing and Outreach: Waiting until the grand opening to start marketing activities. Building brand awareness, establishing referral networks with local physicians, and engaging the community should begin at least 6-9 months before launch.
- Not Investing in Technology from Day One: Skimping on a robust Hospital Information System (HIS) and Electronic Health Record (EHR) system from the start. A good software backbone is essential for efficient operations, data management, and future scalability.
Frequently Asked Questions (FAQ)
Q1: What is the minimum investment to start a small-scale oncology day-care (chemotherapy only) center in a Tier-2 Indian city?
A1: A smaller, specialized day-care setup can significantly reduce the initial capital expenditure by avoiding the high costs of radiation equipment, advanced imaging, and extensive construction. The estimated investment would typically range from ₹2 Crore to ₹5 Crore. This would primarily cover:
1. Facility rent/leasehold improvements.
2. Interior setup with infusion chairs, pharmacy, and consultation rooms.
3. Basic laboratory equipment for blood counts.
4. Licensing and initial working capital.
This model offers a faster path to market and profitability but has a more limited service offering.
Q2: How long does the entire process take, from concept to first patient, in India?
A2: For a comprehensive oncology center involving new construction and radiation facilities, a realistic timeline is 24 to 36 months. The critical path is often determined by two factors:
Construction: Land acquisition, architectural design, and construction can take 18-24 months.
Regulatory Approvals: This is the most unpredictable variable. Securing all approvals, especially the multi-stage clearance from the AERB for radiation facilities, can take 12-18 months and often runs in parallel with construction. Delays here are common and must be factored into the project plan.
Q3: Is NABH accreditation mandatory to open an oncology center in India?
A3: Legally, you can open a facility by registering under the Clinical Establishment Act of your state. However, from a practical and business standpoint, NABH accreditation is virtually essential. Here’s why:
Insurance Empanelment: Most major insurance companies, Third-Party Administrators (TPAs), and corporate clients will only empanel NABH-accredited hospitals. Without this, you lose access to a huge segment of patients with health insurance.
Government Schemes: Empanelment for key government health schemes like CGHS, ECHS, and state-sponsored insurance programs almost always requires NABH accreditation.
Quality Benchmark: It serves as a trusted mark of quality and patient safety, which is a powerful marketing tool and helps build patient confidence.
Q4: Can I get a single-window clearance for all licenses in India?
Unfortunately No. You must apply to multiple, separate authorities at different levels of government:
Local: Municipal Corporation for building plans, Fire Department for NOC.
State: State Pollution Control Board, State Drug Controller, State Health Department (for Clinical Establishment Act).
Central: Atomic Energy Regulatory Board (AERB) for radiation safety.
Due to this complexity, hiring an experienced healthcare consultant or a dedicated legal/liaison team to manage this process is not just a recommendation; it’s a necessity for timely project completion.
Conclusion: Building the Future of Cancer Care in India
Launching an oncology center in India is a marathon, not a sprint. It is an ambitious undertaking that demands significant capital, resilience, and an intimate understanding of a complex market. As we have explored, success is not merely about constructing a building and filling it with equipment. It hinges on the meticulous integration of three foundational pillars:
- A Data-Driven Business Strategy: Grounded in a thorough feasibility study of the local market, a realistic financial model denominated in Indian Rupees, and a clear path to profitability and sustainable ROI.
- Meticulous Regulatory Diligence: Proactively and patiently navigating the labyrinth of central, state, and local regulations, with a particular focus on the long-lead-time approvals from bodies like the AERB, and pursuing quality accreditations like NABH as a strategic imperative.
- An Unwavering Commitment to Clinical Excellence: Building a patient-centric facility, procuring technology that enables high-quality care, and—most importantly—assembling and retaining a skilled, collaborative multidisciplinary team.
The challenges of launching a cancer facility are undeniable, but the opportunity is immense. Rising demand, advanced medical technology, and supportive policies create the right climate for entrepreneurs exploring how to start an oncology center. Beyond financial rewards, such projects bridge critical healthcare gaps and bring hope and healing to thousands of families across India—shaping the future of cancer care.
“Modern oncology centers rely heavily on advanced imaging. A PET-CT scanner is often central to staging and treatment planning — check our detailed PET CT scan guide to understand costs, workflows, and clinical uses. Additionally, for radionuclide imaging, the gamma camera explanation article provides a complete breakdown of how scintillation cameras power nuclear medicine in oncology settings.”
