From Data Chaos to Clinical Clarity: The Dawn of the Digital ICU
For nearly two decades, I’ve worked at the intersection of medicine and technology, witnessing the controlled chaos of the ICU firsthand. Clinicians face relentless pressure as data pours in from ventilators, monitors, and infusion pumps—often fragmented and siloed—forcing care teams into a reactive mode. But what if we could anticipate deterioration hours before it occurs?
This is the promise of the Digital ICU. More than bedside computers, it represents a fundamental shift—a cyber-physical ecosystem where real-time patient data is continuously integrated, intelligently analyzed, and converted into actionable insights. Accelerated by the COVID-19 pandemic, the Digital ICU is moving critical care from reaction to prediction and precision.
In this analysis, we unpack the Digital ICU landscape—from Patient Data Management Systems (PDMS) to AI-driven predictive models. Through real-world use cases, ROI insights, and implementation challenges, this article offers an evidence-based view of how the Digital ICU is redefining the future of critical care for clinicians, leaders, innovators, and students alike.
Table of Contents
The Technological Backbone of the Digital ICU
The Digital ICU is built upon a layered architecture of interconnected technologies. Each component plays a critical role in capturing, processing, and leveraging the massive volumes of patient data to improve care. As a biomedical engineer, I see this as a complex but elegant system integration challenge, where the whole is far greater than the sum of its parts.
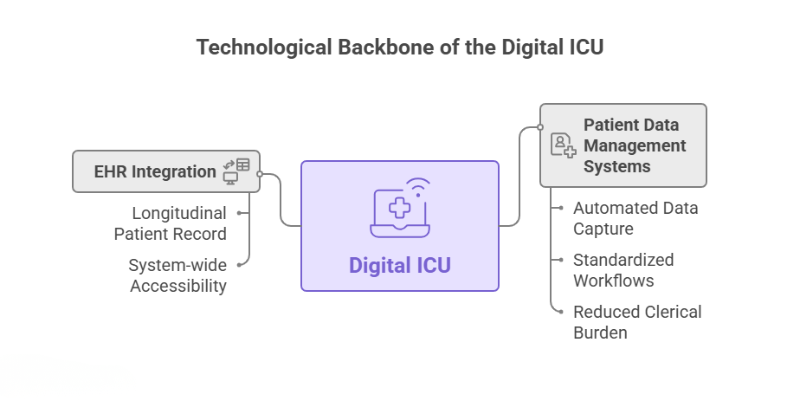
Patient Data Management Systems (PDMS) and EHR Integration
At the core of the Digital ICU is the Patient Data Management System (PDMS), a specialized platform designed for the high-acuity environment. Unlike a standard Electronic Health Record (EHR), a PDMS is built to automatically capture high-frequency data in near real-time from bedside medical devices like monitors and ventilators [3]. This automated data collection is a game-changer. A 2025 study on a PDMS implementation in a trauma ICU highlighted several key features:
- Automated Data Capture: Vital signs and device parameters are imported directly, eliminating manual transcription errors. One study found that an EMR-embedded quality control system reduced false vital-sign entries from 9% to just 1.33% [3, 4].
- Standardized Workflows: The PDMS integrated pre-formatted order sets for medications and nursing care, with features like automatic weight-based dose adjustments and safety alerts for renal impairment.
- Reduced Clerical Burden: By automating documentation, these systems free up significant time for clinicians. A Turkish study found that digitalizing nursing documentation saved an average of 56 minutes per patient per day, allowing more time for direct patient care [1].
The true power of a PDMS is realized when it’s seamlessly integrated with the hospital’s main EHR, such as those from vendors like Epic or Oracle Health (formerly Cerner). This integration ensures that the high-resolution data from the ICU contributes to a complete, longitudinal patient record, accessible across the entire healthcare system [5].
As hospitals become digital ecosystems, the ICU functions as part of a connected smart hospital where real-time data, AI, and advanced platforms drive faster, predictive clinical decisions—an approach already seen in leading Smart Hospitals in Singapore.
The Role of AI and Machine Learning: Predictive Analytics in Action
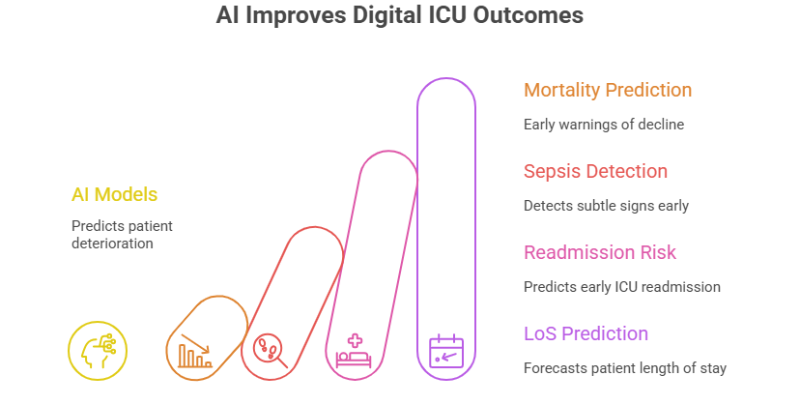
If PDMS is the nervous system of the Digital ICU, then Artificial Intelligence (AI) and Machine Learning (ML) are its brain. By applying algorithms to the vast datasets collected, AI is transforming diagnostics, prognostication, and workflow management [6].
Recent studies have demonstrated the superiority of ML models over traditional scoring systems. For instance, a South Korean study found that ML models (like XGBoost and LightGBM) predicted in-hospital mortality with an Area Under the Receiver Operating Characteristic Curve (AUROC) of up to 0.977, far surpassing conventional models like APACHE III (AUROC 0.77–0.80) [7, 8].
Key applications of AI in the Digital ICU include:
- Mortality and Deterioration Prediction: Models analyze real-time variables like ventilator use, glucose levels, and infection indicators to provide early warnings of patient decline [8].
- Sepsis Detection: AI platforms can detect subtle signs of sepsis hours before they become clinically apparent, enabling life-saving early intervention [9].
- Readmission Risk: Optimized ML models can predict early ICU readmission with high accuracy (AUROC of 0.92), identifying at-risk patients before they are discharged from the unit. Key predictors include ICU length of stay (LoS), PaO₂ levels, and white blood cell counts [1, 8].
- Length of Stay (LoS) Prediction: Algorithms help forecast patient LoS, enabling better resource management and patient flow optimization [1].
Internet of Things (IoT) and Continuous Monitoring
The Internet of Things (IoT) in healthcare refers to the network of connected devices, wearables, and sensors that continuously collect physiological data. In the Digital ICU, this includes:
- Wearable Biosensors: Devices that track heart rate, respiratory rate, temperature, and SpO2 continuously, providing a much richer dataset than intermittent spot checks [10].
- Continuous Glucose Monitors (CGM): Crucial for managing glycemic control in critically ill patients, CGM devices have been shown to improve time spent in the normal blood glucose range and reduce hyperglycemic events [4].
- Smart Beds: Beds with integrated sensors can monitor patient movement and pressure distribution, helping to prevent pressure ulcers. One study using a continuous bedside pressure mapping (CBPM) system saw a significant decrease in the development of stage II pressure ulcers [4].
This constant stream of data from IoT devices feeds the PDMS and AI models, creating a high-resolution, dynamic picture of the patient’s condition and enabling a truly proactive approach to care [10].
Tele-ICU and Remote Command Centers
The Tele-ICU, or virtual ICU, model uses technology to extend the reach of critical care specialists (intensivists) to multiple locations from a central command center. This is particularly vital for rural or underserved hospitals that lack 24/7 on-site intensivist coverage [11]. Enabled by high-speed connectivity like 5G, Tele-ICU platforms facilitate:
- Real-time Remote Monitoring: A remote team of intensivists and critical care nurses can monitor patient data, view high-quality video feeds, and collaborate with bedside staff [11].
- Expert Consultations: Bedside teams can instantly access specialist expertise for complex cases or emergencies.
- Standardization of Care: Tele-ICU programs help enforce evidence-based best practices and protocols across multiple hospitals, improving overall quality of care [4].
This model not only bridges gaps in care but has also proven to be a scalable and cost-effective solution for optimizing critical care resources [11, 12].
Scientific Deep Dive: How Predictive Analytics Work in the Digital ICU
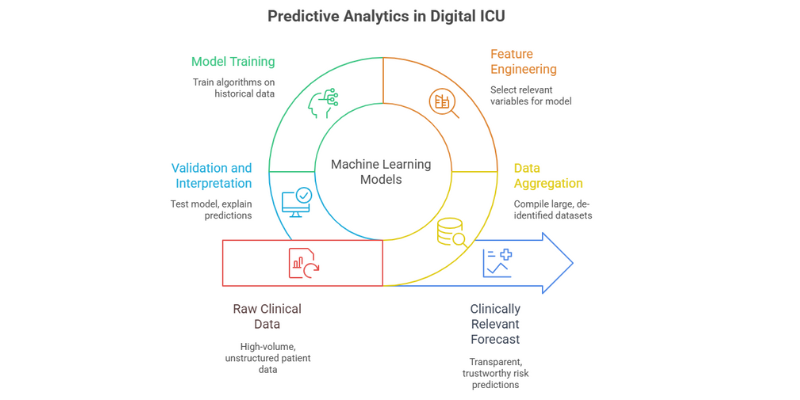
The “magic” of the Digital ICU’s predictive power isn’t magic at all; it’s the result of sophisticated machine learning models trained on massive clinical datasets. As an engineer, I find the mechanics of this process fascinating. It involves taking the raw, high-volume data captured by the PDMS and transforming it into a clinically relevant forecast.
The process generally follows these steps:
- Data Aggregation: Large, de-identified datasets are compiled. A famous example is the MIMIC (Medical Information Mart for Intensive Care) database, which contains data from tens of thousands of ICU stays and is a cornerstone for much of the research in this field [1, 8].
- Feature Engineering: Researchers and data scientists select the most relevant variables (features) from the dataset. For predicting mortality, a model might be trained on over 70 variables, including demographics, lab results (glucose, white blood cells), vital signs, and device settings (ventilator use, PaO₂) [7, 8].
- Model Training: Algorithms like XGBoost (Extreme Gradient Boosting) and Random Forest are “trained” on this historical data. The model learns the complex, non-linear patterns and correlations between the input features and the outcome of interest (e.g., survival, readmission).
- Validation and Interpretation: The model’s performance is tested on a separate dataset it has never seen before to ensure it can generalize to new patients. This is a critical step, as models trained on single-center data can sometimes perform poorly in different clinical environments—a major challenge in the field [8]. To combat the “black box” problem, where clinicians can’t see *why* a model made a certain prediction, techniques like SHAP (Shapley Additive Explanations) are used. SHAP helps explain which features (like a rising white blood cell count) contributed most to a specific risk score, making the AI’s output more transparent and trustworthy for clinicians [1].
By identifying critical thresholds for these parameters, the model enabled the configuration of alarms for high-risk patients, facilitating timely interventions. This underscores the transformative potential of explainable AI in critical care by supporting clinicians with actionable insights [1].
Use Cases & Real-World Applications: The Digital ICU in Action
Theory and research are vital, but the true test of any technology is its impact in the real world. Across the globe, hospitals are implementing components of the Digital ICU and reporting transformative results. Here are a few evidence-backed examples.
Case Study 1: AI-Powered Sepsis Detection at Cleveland Clinic
Sepsis, a life-threatening response to infection, is a leading cause of hospital deaths. Early detection is critical. In 2025, Cleveland Clinic expanded its use of Bayesian Health’s AI platform for sepsis detection across its hospital network. The platform, which is integrated with the EHR, continuously analyzes patient data for subtle signs of impending sepsis [9].
- Results: In a pilot study, the AI model improved the detection of sepsis cases by 46% compared to legacy rule-based alerts. Crucially, it reduced the number of false alerts by a factor of 10, combating the pervasive problem of alert fatigue. Alerts were generated an average of 6-7 hours before antibiotic treatment was initiated, giving clinicians a critical window for intervention [9].
- Impact: The underlying AI model had previously demonstrated an 18% relative reduction in mortality in FDA trials. This case study is a powerful example of how AI can augment clinical awareness and directly save lives [9].
Case Study 2: PDMS Implementation and Infection Control
A 2025 single-center study by Toma et al. provides a granular look at the impact of transitioning from paper-based charting to a comprehensive PDMS in a trauma ICU. The intervention focused on changing the documentation infrastructure, not clinical protocols [3].
- Results: Post-digitalization, the median ICU length of stay was significantly reduced from 13.0 days to 6.0 days. While adjusted mortality rates did not reach statistical significance, they showed a favorable downward trend (18.6% to 6.2%). Furthermore, there were statistically significant reductions in per-patient rates of ventilator-associated pneumonia (VAP) and bloodstream infections (BSI) [3].
- Impact: This study demonstrates that even a foundational step like implementing a PDMS can lead to tangible improvements in efficiency and patient safety, likely by improving data fidelity, facilitating adherence to care bundles, and enabling better tracking of device-days [3].
Case Study 3: The Financial and Clinical Case for Tele-ICU
Tele-ICU programs have consistently demonstrated a strong return on investment. A study by the UMass Memorial Critical Care Operations Group compared a facility before and after implementing Tele-ICU services. The results were striking:
- A 20% increase in case volume.
- A 9% reduction in overall hospital length of stay.
- A 294% increase in the direct contribution margin per case [12].
Even in smaller settings, the benefits are clear. A 60-bed military hospital, General Leonard Wood Army Community Hospital, implemented a Tele-ICU and saw a 30% reduction in costs associated with transferring patients to outside facilities and achieved a 19% ROI in the first year [12]. These cases show that the Digital ICU isn’t just for large academic centers; its principles can be scaled to benefit diverse healthcare environments.
The ROI of Digital Transformation: A Multi-Faceted Analysis
For hospital administrators and policymakers, the decision to invest millions in a Digital ICU transformation hinges on the return on investment (ROI). This return isn’t purely financial; it’s a composite of clinical, operational, and financial gains that collectively build a powerful business case.
Clinical ROI: Better Outcomes, Safer Care
The primary driver for any healthcare investment must be patient well-being. The Digital ICU delivers on this front by measurably improving key quality indicators.
- Reduced Mortality: The integration of EHRs and Computerized Physician Order Entry (CPOE) has been linked to a 12% reduction in ICU mortality rates, attributed to fewer medical errors and better decision-making [1].
- Fewer Readmissions: Remote Patient Monitoring (RPM) programs for high-risk patients have been shown to lower 30-day readmission rates by as much as 76% [13].
- Lower Infection Rates: As seen in the PDMS case study, digitalization is associated with significant reductions in device-associated infections like VAP and BSI [3].
- Shorter Length of Stay (LoS): Digital dashboards and predictive analytics help streamline care and discharge planning, with studies showing LoS reductions from 0.28 days to as much as 18.8 days in some cases [14].
Operational ROI: Efficiency, Workflow, and Staff Satisfaction
An efficient hospital is a safer and more financially sound hospital. The Digital ICU streamlines operations and, crucially, addresses a major driver of cost and turnover: staff burnout.
- Time Savings: Automating documentation saves immense amounts of time. As noted, Turkish nurses saved 56 minutes per patient daily [1]. AI scribes, trialed at Atrium Health, led to 43% of physicians reporting less documentation time and 44% feeling less EHR-related frustration [9].
- Improved Resource Allocation: Predictive analytics for patient flow, bed management, and staffing lead to more efficient use of expensive ICU beds and personnel [1].
- Reduced Errors: Automated Drug Dispensing Systems (ADDS) have been shown to significantly reduce medication error rates (from 18.6% to 13.5% in one study), and CPOE systems prevent errors related to illegible handwriting or incorrect dosing [4].
ROI is often cost avoidance, not just cost savings. And some benefits, like improving staff experience, are just the cost of doing business. These “soft” benefits—reduced frustration, better team collaboration—are critical and can be measured through staff satisfaction surveys and retention rates [15].
Financial ROI: The Bottom Line
While clinical and operational improvements are valuable, they also translate directly into financial gains. Calculating a precise ROI requires a detailed analysis of costs versus benefits.
Costs:
- Technology & Infrastructure: This is the largest expense. EHR implementation can be massive, with vendors like Epic having higher upfront costs than Oracle Health (Cerner) [16, 17]. A full Tele-ICU implementation can cost $2-5 million to set up, with annual operating costs of $600k to $1.5 million [18]. A VHA analysis found a total first-year cost of about $123,000 per ICU bed [19].
- Staffing & Training: Significant investment is needed for training, change management, and potentially hiring new staff like data analysts or “digital champions” to drive adoption [20, 21].
- Ongoing Maintenance: Software licenses, support contracts, and hardware upgrades are recurring costs.
Benefits (Revenue & Savings):
- Increased Revenue: Tele-ICU can increase case volume and per-case revenue [12]. RPM programs can generate significant new revenue streams through established CPT codes, with providers earning $120+ per patient per month [22].
A simple ROI calculation [(Total Benefits – Total Costs) / Total Costs] can provide a snapshot, but a full Net Present Value (NPV) analysis is better for long-term strategic decisions. The evidence strongly suggests that while the initial investment is substantial, the financial returns are rapid and significant [24].
A Balanced Perspective: The Promise and Perils of the Digital ICU
No technological revolution is without its challenges. As a biomedical engineer, I’m trained to be both an optimist about innovation and a pragmatist about implementation. A successful Digital ICU strategy requires a clear-eyed view of both the benefits and the barriers.
The Pros: A Paradigm Shift in Critical Care
- Proactive, Predictive Care: Moves from reacting to crises to predicting and preventing them, fundamentally improving patient outcomes [1].
- Enhanced Patient Safety: Drastically reduces medication errors, hospital-acquired infections, and other adverse events through automation and decision support [3, 4].
- Optimized Resource Utilization: Smart allocation of beds, staff, and equipment improves hospital throughput and reduces waste [1].
- Reduced Clinician Burnout: Automating administrative tasks like documentation frees clinicians to focus on patient care, improving job satisfaction and retention [9, 20].
- Democratized Expertise: Tele-ICU and remote monitoring extend the reach of specialist care, improving health equity for rural and underserved populations [11].
- Improved Family Engagement: Digital tools like virtual family meetings and ICU diaries keep loved ones informed and involved, improving satisfaction for all [2, 25].
The Cons: Significant Implementation Hurdles
- High Upfront Cost & Complexity: The financial investment in infrastructure, software, and training is substantial and can be a major barrier for many institutions [18, 21].
- Interoperability Challenges: Getting disparate systems (EHRs, PDMS, lab systems, devices) to communicate seamlessly remains a major technical hurdle [20].
- Clinician Resistance & Usability: Poorly designed systems with cumbersome workflows can increase frustration and lead to low adoption. A study on a CCIS found that while clinicians preferred it to paper, major usability issues remained, including excessive clicks and disorganized layouts [20].
- Alert Fatigue: An overabundance of alarms and notifications from predictive models can lead to clinicians ignoring them, negating their benefit [3].
- Data Security and Privacy: Centralizing vast amounts of sensitive patient data creates a high-value target for cyberattacks and requires robust security measures [3].
- AI “Black Box” and Generalizability: Lack of transparency in some AI models hinders clinical trust. Furthermore, models trained on one hospital’s population may not work well in another, requiring extensive local validation [8].
Common Questions & FAQs
What’s the real difference between a Digital ICU and a regular ICU with computers?
It’s a difference in integration and intelligence. A regular ICU might have computers for EHR access, but the data is often entered manually and systems don’t talk to each other. A Digital ICU is an integrated ecosystem. It automatically captures data from bedside devices in real-time, centralizes it in a PDMS, and uses AI to analyze that data for predictive insights. It’s the shift from a digital filing cabinet to an intelligent, proactive co-pilot for the clinical team.
How much does it cost to implement a Digital ICU?
Costs vary widely based on scale and existing infrastructure. A comprehensive Tele-ICU implementation can cost $2-5 million upfront with over $1 million in annual operating costs [18]. A detailed VHA analysis pegged the first-year cost at around $123,000 per ICU bed [19]. However, the ROI is often rapid and substantial. For example, RPM programs can be implemented with per-patient-per-month software fees and generate immediate revenue, while larger AI platform investments can yield millions in cost savings from reduced LoS and complications.
Will AI replace ICU clinicians?
Absolutely not. The goal of AI in the ICU is to augment, not replace, human expertise. AI is a powerful tool for detecting patterns in data that are invisible to the human eye, but it lacks the clinical judgment, empathy, and contextual understanding of an experienced doctor or nurse. The vision is for AI to handle the cognitive heavy lifting of data analysis and administrative tasks, freeing clinicians to focus on what they do best: complex decision-making and direct patient care.
How do you ensure patient data is secure in a Digital ICU?
Security is paramount. A multi-layered approach is essential. This includes robust network security (firewalls, encryption of data in transit and at rest), strict access controls (individual authentication, role-based permissions), and compliance with regulations like GDPR and HIPAA. All patient data must be stored on secure hospital servers, and any data used for research must be thoroughly de-identified.
Our hospital has an EHR. Isn’t that enough for a Digital ICU?
An EHR is a necessary foundation, but it’s not sufficient for a true Digital ICU. Standard EHRs are not typically designed to handle the high-frequency, real-time data streams from critical care devices. A specialized Patient Data Management System (PDMS) or Clinical Information System (CIS) is needed to capture and manage this granular data. The PDMS then integrates with the EHR to provide a complete picture. Think of the EHR as the patient’s long-term biography and the PDMS as the minute-by-minute diary of their ICU stay.
What is the first step to starting a digital transformation in our ICU?
Start with a clear strategy and a needs assessment. Don’t chase shiny technology. Instead, identify the biggest pain points in your ICU. Is it staff burnout from documentation? High infection rates? Delayed discharges? Once you have a clear problem to solve, you can identify the right technology to address it. A crucial first step is to engage frontline clinicians—nurses, physicians, respiratory therapists—in the process. Their buy-in and insights are essential for success. Starting with a focused pilot project is often the best approach to demonstrate value and build momentum.
Conclusion: The Future is Data-Driven and Human-Centered
The Digital ICU represents one of the most significant advancements in critical care in a generation. By harnessing the power of real-time data, integrated systems, and artificial intelligence, we are moving away from a reactive model of care and toward a future that is predictive, precise, and personalized. The evidence is clear: this transformation leads to better patient outcomes, more efficient hospitals, and a more sustainable work environment for our invaluable clinical teams.
However, the path to a fully realized Digital ICU is paved with challenges. It requires substantial financial investment, a robust technical infrastructure, and, most importantly, a cultural shift. Success is not guaranteed by technology alone. It is achieved through strategic planning, a relentless focus on user-centric design, and a commitment to training and supporting the people who will use these new tools.
As a biomedical engineer who has spent a career bridging the gap between technology and medicine, my actionable advice for any institution considering this journey is this:
- Start with “Why”: Define the clinical or operational problem you are trying to solve before you select the technology.
- Build a Multidisciplinary Team: Involve clinicians, IT, administrators, and biomedical engineers from day one. User involvement is not optional; it is the single most important predictor of success [21].
- Think Big, Start Small: Have a long-term vision for your Digital ICU, but begin with a manageable pilot project to prove value and secure buy-in.
- Measure Everything: Establish clear metrics for clinical, operational, and financial ROI from the outset to justify the investment and track progress.
The Digital ICU is here. It is no longer a futuristic concept but a present-day reality that is saving lives and reshaping our healthcare systems for the better. The journey is complex, but the destination is worth it.
