Introduction
Kidney disease is a silent epidemic affecting millions worldwide. When kidneys fail, the body’s ability to filter waste products and excess fluids from the blood is compromised, leading to life-threatening complications. Globally, over 2 million people rely on dialysis or a kidney transplant to manage kidney disease (Cleveland Clinic). At the heart of this life-sustaining treatment for many is the remarkable dialysis machine. This blog post aims to demystify the dialysis machine, exploring its intricate workings, its profound medical importance in managing kidney failure, and the exciting technological advancements shaping its future. We will journey from a foundational understanding of kidney failure to a deep dive into the technical operation of these vital devices, their medical significance, and the cutting-edge innovations that promise a brighter future for patients.A modern dialysis machine, a beacon of hope for patients with kidney failure.
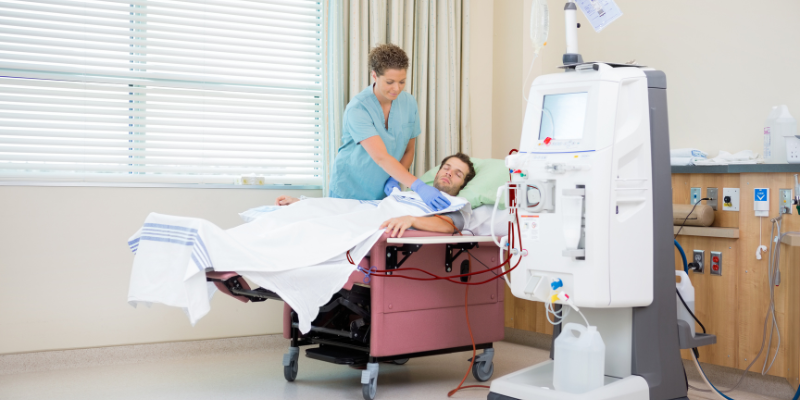
Table of Contents
Understanding Kidney Failure: The Imperative for Dialysis
What is Kidney Failure?
Kidney failure, also known as End-Stage Renal Disease (ESRD), is the most advanced stage of chronic kidney disease. It occurs when the kidneys lose most of their ability to function, typically operating at less than 15% of their normal rate (Cleveland Clinic). In this state, the kidneys can no longer effectively filter waste products (like urea and creatinine) and excess fluids from the blood. Common causes leading to kidney failure include diabetes and high blood pressure, though other conditions like lupus or genetic factors can also contribute (Cleveland Clinic). If you want to know more about kidney disease symptoms, click here.
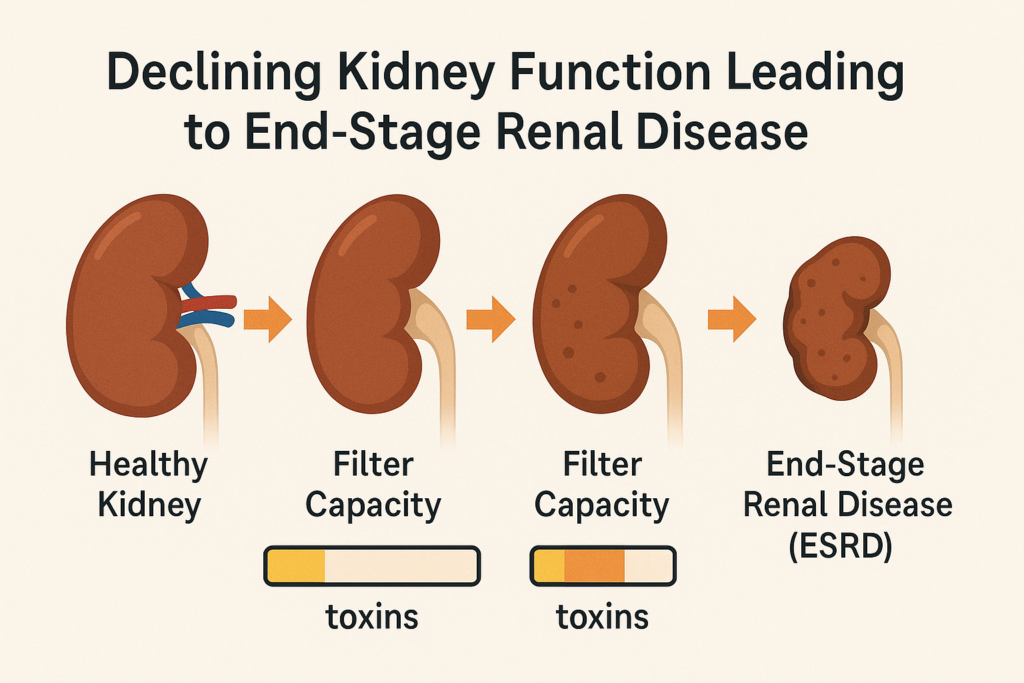
Conceptual illustration of declining kidney function leading to End-Stage Renal Disease (ESRD).
Why Dialysis Becomes Essential
When kidney function declines to the point of failure, toxins and excess fluid accumulate in the bloodstream, a condition known as uremia. This buildup can lead to severe health problems, including high blood pressure, anemia, bone disease, nerve damage, and eventually, if untreated, death. Dialysis becomes essential as it takes over the critical functions of the failing kidneys, removing waste products, balancing electrolytes, and eliminating excess fluid from the body (Cleveland Clinic). Without treatment like dialysis or a kidney transplant, kidney failure is fatal, with survival typically limited to a few days or weeks (Cleveland Clinic).
Key Takeaways: Understanding Kidney Failure
- Kidney failure (ESRD) signifies that kidneys function at less than 15% of their normal capacity.
- Common causes include diabetes and hypertension.
- Dialysis is crucial to remove toxins and excess fluid, preventing life-threatening complications when kidneys fail.
How Dialysis Machines Work: A Technical Deep Dive
A dialysis machine is a sophisticated medical device engineered to replicate the essential filtration functions of healthy kidneys. While there are different types of dialysis, the machine itself is most prominently associated with hemodialysis.
Types of Dialysis
There are two primary types of dialysis:
- Hemodialysis (HD): This is the most common type of dialysis. It involves using a dialysis machine and an external filter, called a dialyzer (or artificial kidney), to clean the blood. Blood is removed from the body, filtered through the dialyzer, and then returned to the body (Cleveland Clinic). Hemodialysis can be performed in a dialysis center or at home.
- Peritoneal Dialysis (PD): This type uses the lining of the patient’s abdomen (peritoneum) and a special solution called dialysate to filter blood. The dialysate is introduced into the abdominal cavity, where it draws waste products and excess fluid from the blood vessels in the peritoneum. After a certain period, the used dialysate is drained (Cleveland Clinic). While PD doesn’t use an external “dialysis machine” in the same way as HD, automated PD (APD) utilizes a machine called a cycler to manage the exchange of dialysate, often while the patient sleeps.
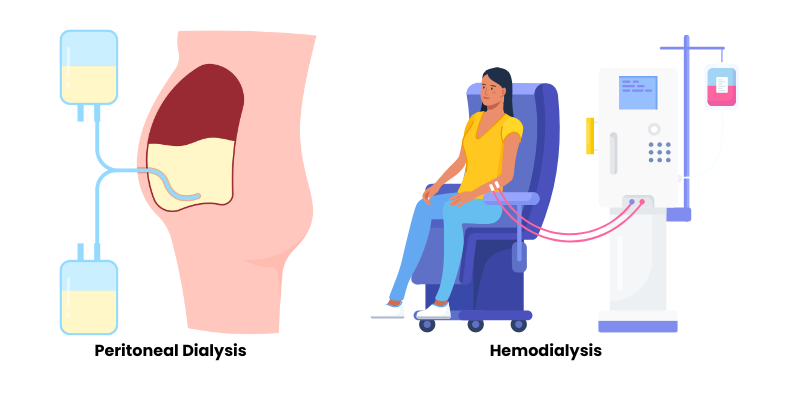
Conceptual distribution of dialysis treatment modalities.
The Hemodialysis Machine in Detail
The hemodialysis machine is a complex system with several key components working in concert to ensure safe and effective blood purification.
1. Blood Circuit
The blood circuit is responsible for transporting the patient’s blood to the dialyzer and back to the patient. It consists of:
- Blood Lines: Sterile tubing that carries blood from the patient’s vascular access (e.g., a fistula or graft in the arm) to the dialyzer (arterial line) and from the dialyzer back to the patient (venous line) (Medmastery).
- Blood Pump: This peristaltic pump draws blood from the patient and pushes it through the dialyzer at a controlled rate, typically 200-500 mL/minute (Natron Equipments). The pump segment of the arterial blood line is threaded through the pump.
- Heparin Pump: To prevent blood from clotting as it passes through the extracorporeal circuit, an anticoagulant like heparin is continuously infused into the blood circuit, usually before the dialyzer. The heparin pump delivers a precise, prescribed dose (DaVita).
2. Dialyzer (Artificial Kidney)
The dialyzer is the heart of the hemodialysis process. It’s a clear plastic cylinder containing thousands of hair-thin hollow fibers made of a semi-permeable membrane (Home Dialysis Central).
- Blood Compartment: Blood flows through the inside of these hollow fibers.
- Dialysate Compartment: Specially prepared dialysis fluid (dialysate) flows around the outside of these fibers in the opposite direction (counter-current flow).
- Filtration Process: Waste products (like urea, creatinine) and excess electrolytes (like potassium, phosphorus) diffuse from the blood across the semi-permeable membrane into the dialysate, driven by concentration gradients. Excess fluid is removed from the blood by ultrafiltration, driven by a pressure gradient created by the machine. Proteins and blood cells, being larger, are retained in the blood (NIDDK).
Diagram illustrating the counter-current flow of blood and dialysate within the dialyzer’s hollow fibers.
3. Dialysate Circuit
The dialysate circuit prepares and delivers the dialysis fluid to the dialyzer and disposes of the used fluid.
- Dialysate Composition: Dialysate is a precisely formulated solution containing purified water, electrolytes (like sodium, potassium, calcium, magnesium, chloride), and bicarbonate (to buffer acids in the blood). The machine mixes an acidified concentrate and a bicarbonate concentrate with purified water to create the final dialysate (DaVita). The nephrologist prescribes the specific dialysate composition based on the patient’s needs.
- Monitoring and Control: The dialysis machine continuously monitors and controls the dialysate’s temperature, conductivity (to ensure correct mixture), and flow rate. It also checks for blood leaks from the dialyzer into the dialysate.
- Degassing and Heating: Water used for dialysate is degassed to prevent air bubbles in the circuit and heated to body temperature for patient comfort and to optimize diffusion.
4. Safety Mechanisms and Monitoring
Hemodialysis machines are equipped with numerous safety features and alarms to protect the patient:
- Pressure Monitors: Arterial and venous pressure monitors detect issues with blood flow, such as kinks in the lines or clotting in the dialyzer.
- Air Detection: Air traps and ultrasonic air detectors are present in the venous line to prevent air embolisms. If air is detected, the blood pump stops, and the venous line is clamped (DaVita).
- Blood Leak Detector: An optical sensor checks the used dialysate for traces of blood, which would indicate a rupture in the dialyzer membrane.
- Temperature and Conductivity Sensors: Ensure the dialysate is correctly mixed and at the right temperature.
- Alarms: If any parameter goes out of the safe range, an alarm sounds, and often, the machine will enter a bypass mode or stop treatment until the issue is resolved (DaVita).
The Process of Hemodialysis: A Step-by-Step Overview
- Preparation: The patient is weighed, and vital signs are taken. The vascular access site is cleaned.
- Connection: Two needles are inserted into the patient’s vascular access. One needle (arterial) draws blood to the dialysis machine, and the other (venous) returns the cleaned blood (NIDDK). These needles are connected to the blood lines.
- Initiation: The blood pump is started, and heparin infusion begins. Blood circulates through the dialyzer.
- Treatment: For 3-5 hours (typical session length), the machine continuously pumps blood through the dialyzer, while dialysate flows on the other side of the membrane, cleaning the blood. The machine monitors all critical parameters.
- Discontinuation: Once the prescribed treatment time is complete, the blood pump is stopped. The blood in the extracorporeal circuit is rinsed back to the patient using saline.
- Disconnection: Needles are removed, and pressure is applied to the access sites to stop bleeding. Post-treatment weight and vital signs are recorded.
Key Takeaways: How Dialysis Machines Work
- Hemodialysis machines use a blood circuit to move blood, a dialyzer (artificial kidney) for filtration, and a dialysate circuit to provide the cleaning solution.
- The dialyzer contains semi-permeable membranes allowing waste and excess fluid removal via diffusion and ultrafiltration.
- Sophisticated monitoring systems and alarms ensure patient safety throughout the treatment.
- The process involves connecting the patient to the machine, circulating and cleaning blood for several hours, and then returning the blood.
Medical Importance and Patient Experience
The dialysis machine is more than just a piece of equipment; it’s a lifeline for individuals with ESRD. Its medical importance cannot be overstated, profoundly impacting survival and quality of life, though the experience itself presents unique challenges.
The Lifesaving Role of Dialysis
For patients whose kidneys have failed, dialysis performs essential life-sustaining functions. It removes metabolic waste products, excess water, and helps balance electrolytes and blood pressure (NIDDK). While not a cure for kidney failure, dialysis allows patients to live longer and feel better, often serving as a bridge to kidney transplantation or as a long-term therapy for those not eligible for a transplant. Without dialysis, ESRD is fatal (Cleveland Clinic).
Improving Quality of Life (QoL)
By alleviating the symptoms of uremia (such as fatigue, nausea, and swelling), dialysis can significantly improve a patient’s energy levels and overall well-being. Effective dialysis allows patients to continue engaging in daily activities, work, and social interactions. However, the impact on QoL is complex and varies greatly among individuals, influenced by the type of dialysis, treatment schedule, and overall health.
Patient Experience and Challenges
Living with dialysis involves significant lifestyle adjustments and can present various challenges:
- In-Center Hemodialysis:
- Time Commitment: Typically requires 3 sessions per week, each lasting 3-5 hours, plus travel time to and from the dialysis center (NIDDK). This fixed schedule can impact work and personal life.
- Physical Discomfort: Needle insertions can be uncomfortable, though numbing creams can help (Kidney.org). Some patients experience side effects like cramping, fatigue, or hypotension during or after treatment.
- Dietary and Fluid Restrictions: Patients often need to follow strict dietary and fluid intake guidelines.
- Psychological Impact: Dependence on a machine and the chronic nature of the illness can lead to emotional and psychological stress. However, studies show that factors like competent and empathetic nursing staff, positive peer interactions, and family support significantly contribute to patient comfort and a better experience (Patients’ Perception of Comfort Facilitators During Hemodialysis – PMC).
- Home Hemodialysis (HHD) and Peritoneal Dialysis (PD):
- Increased Flexibility and Autonomy: HHD and PD offer more flexible schedules and can be performed in the comfort of one’s home, potentially leading to better QoL (NIDDK). More frequent or longer HHD sessions can lead to better clinical outcomes and fewer dietary restrictions.
- Responsibility and Training: These modalities require significant patient and/or caregiver training and responsibility for managing treatment, supplies, and potential complications. Fear of performing dialysis at home and limited space can be barriers (Identifying Major Barriers to Home Dialysis – AJKD – Note: Full text access may be required).
- Shared Hemodialysis Care (SHC): This model involves patients becoming active partners in their in-center treatment, learning to perform tasks like setting up the machine or self-cannulation. SHC is associated with better physiological, psychological, and social outcomes, increased patient activation, and can be a stepping stone to home dialysis (Fresenius Medical Care).
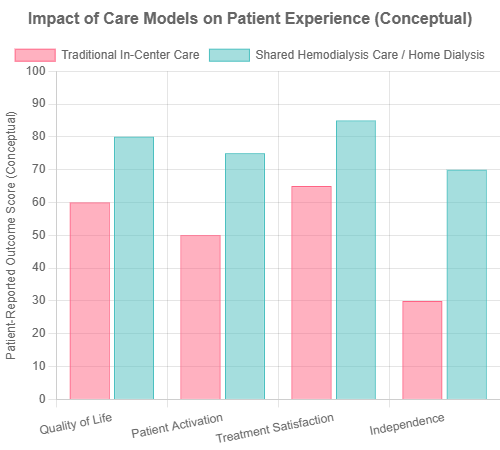
Conceptual comparison of patient-reported outcomes in Traditional vs. Shared Hemodialysis Care.
Key Takeaways: Medical Importance and Patient Experience
- Dialysis machines are lifesaving, performing vital kidney functions for ESRD patients.
- While improving QoL by reducing uremic symptoms, dialysis imposes significant lifestyle changes and challenges.
- In-center HD involves time commitment and potential discomfort, while home therapies (HHD, PD) offer flexibility but require patient responsibility.
- Shared Hemodialysis Care can empower patients and improve outcomes.
- Patient comfort is enhanced by competent staff, peer support, and family involvement.
Technological Advancements in Dialysis
While conventional dialysis has been a mainstay for decades, the field is continuously evolving. The drive for innovation focuses on making dialysis more efficient, portable, patient-friendly, and ultimately, to better mimic natural kidney function (Advances in hemodialysis therapy – PMC).
The Drive for Innovation
Current renal replacement therapies primarily address the filtration function of the kidneys but fall short in replacing their metabolic, endocrinologic, and immunologic roles. Limitations also include the lack of portability and the burden of treatment schedules (PMC10241346). Thus, research is geared towards total replacement, enhanced portability, improved quality of life, and reduced economic impact.
Portable Hemodialysis Machines
One significant advancement is the development of more compact and user-friendly hemodialysis machines for home use. These devices aim to improve patient autonomy and flexibility. Some modern home HD machines feature intuitive interfaces and remote monitoring capabilities, allowing healthcare providers to track patient progress without frequent clinic visits (OCRC.net – snippet, full site failed to load). These machines make daily or nocturnal home hemodialysis more feasible, which can lead to better clinical outcomes.Portable hemodialysis machines are enhancing patient autonomy and treatment flexibility.
Wearable Artificial Kidneys (WAKs)
The concept of a WAK aims to provide continuous or near-continuous dialysis, allowing patients greater mobility and a more normal lifestyle. These devices are typically miniaturized and designed to be worn by the patient.
- Hemodialysis-based WAKs: These devices miniaturize the components of a conventional HD machine, including a small dialyzer and pumps, often incorporating sorbent technology to regenerate a small volume of dialysate. Early prototypes faced technical challenges like clotting, kinking of tubes, and battery issues (PMC10241346). The WAK V1.0 and its successors demonstrated technical breakthroughs like pulsatile flow to enhance clearance (Technical Breakthroughs in the WAK – PMC).
- Peritoneal Dialysis-based WAKs:
- AWAK (Automated Wearable Artificial Kidney): This is a peritoneal dialysis-based system. It’s “bloodless” as it doesn’t require extracorporeal blood circulation. It uses sorbent technology to continuously regenerate and reuse spent peritoneal dialysate, making it “waterless” by eliminating the need for large volumes of fresh dialysate bags (SpringerLink – AWAK). A modified AWAK automates PD by providing small tidal infusions, with a purse-sized controller and a disposable cartridge that rejuvenates dialysate (The Wearable Artificial Kidney – PMC). Preliminary studies have explored its safety and efficacy, noting that while generally safe, some participants experienced abdominal discomfort (PubMed – AWAK Safety Study).
- WEAKID (Wearable Artificial Kidney Device): Another PD-based system using sorbent technology to continuously circulate and refresh peritoneal dialysate. It’s designed for portable (e.g., 8 hours/night) or wearable (e.g., 16 hours/day) dialysis (Artificial kidney: Challenges and opportunities – PMC).
Wearable devices hold the promise of significantly improving patient quality of life by allowing continuous dialysis during daily activities, potentially loosening dietary restrictions and reducing medication burden (Portable and wearable dialysis devices – PMC).
Hemodiafiltration (HDF)
HDF combines the principles of hemodialysis (diffusion) and hemofiltration (convection) to remove a broader range of uremic toxins, including small and middle-sized molecules. Online HDF generates large volumes of sterile substitution fluid online, which is infused into the patient. HDF is postulated to reduce intradialytic hypotension, dialysis-related amyloidosis, and improve erythropoietin responsiveness (PMC10241346). However, its adoption in some regions like the US has been slower due to increased costs, regulatory concerns about online fluid production, and mixed results from clinical trials regarding overall survival benefits (PMC10241346).
Implantable Bioartificial Kidneys (BAK)
Perhaps the most ambitious advancement is the development of an implantable bioartificial kidney. This technology aims to create a fully implantable device that not only filters blood but also replicates other kidney functions like metabolic and endocrine activities. The BAK typically combines a high-efficiency hemofilter with a bioreactor containing cultured renal tubule epithelial cells (Recent innovations in renal replacement technology – PMC). These cells can perform active transport and metabolic functions. While still in preclinical or early clinical trial stages, the BAK represents a potential paradigm shift, moving beyond simple filtration towards true kidney replacement.
Key Takeaways: Technological Advancements
- Innovation aims for more portable, efficient, and patient-friendly dialysis that better mimics natural kidney function.
- Portable HD machines enhance home dialysis feasibility and patient autonomy.
- Wearable Artificial Kidneys (WAKs), both HD and PD-based (like AWAK and WEAKID), promise continuous dialysis and improved mobility, using sorbent technology.
- Hemodiafiltration (HDF) offers enhanced clearance of middle molecules but faces adoption challenges.
- Implantable Bioartificial Kidneys (BAK) are a future goal, combining filtration with cellular bioreactors to replicate broader kidney functions.
The Future of Dialysis & Conclusion
The journey of the dialysis machine from a cumbersome, hospital-bound apparatus to potentially wearable or even implantable devices is a testament to medical and engineering ingenuity. Key trends shaping the future include further miniaturization, enhanced biocompatibility of materials, personalized treatment algorithms leveraging AI, and a greater focus on improving overall patient quality of life, not just survival.
Initiatives like KidneyX (a public-private partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology) are accelerating innovation in the prevention, diagnosis, and treatment of kidney diseases, with a strong emphasis on developing next-generation dialysis technologies (PMC10241346).
In conclusion, the dialysis machine remains a cornerstone in the management of kidney failure, extending and improving the lives of millions. Understanding its technical operation reveals a sophisticated interplay of engineering and physiology. While current technologies have limitations, the continuous advancements in portability, efficiency, and biocompatibility offer hope for a future where dialysis is less burdensome and more integrated into patients’ lives, ultimately paving the way for therapies that can more holistically replace lost kidney function. The dedication of researchers, clinicians, and engineers ensures that the evolution of the dialysis machine will continue to bring tangible benefits to those affected by kidney disease.
