The 2025 Indian Biomedical Engineering Landscape: Innovation in Healthcare
The year 2025 represents a transformative period for biomedical engineering careers in India. No longer merely an emerging field, it has evolved into a cornerstone of India’s healthcare revolution, blending biology, medicine, and engineering to address unique healthcare challenges faced by the world’s second-most populous nation. From AI-powered diagnostic tools designed specifically for rural healthcare settings to affordable medical devices manufactured locally, Indian biomedical engineers are pioneering solutions that are both innovative and contextually relevant.
This dynamic growth is fueled by multiple factors including the government’s push towards an “Atmanirbhar Bharat” (Self-Reliant India) in healthcare technology, increasing healthcare demands of a growing middle class, and the persistent need for affordable medical solutions. The impact of biomedical engineering in India is becoming increasingly significant.
Consider these key statistics: Employment for biomedical engineers in India is projected to grow at 10-12% annually between 2023 and 2028, outpacing many other engineering disciplines. The Indian medical devices market, which directly employs biomedical engineers, is expected to reach $50 billion by 2025, growing at a CAGR of approximately 15% (Invest India Report).
Furthermore, the “Make in India” initiative has spurred tremendous growth in Biomedical Engineering careers in India’s domestic medical device manufacturing, creating thousands of new jobs for biomedical engineers. According to the Association of Indian Medical Device Industry (AiMeD), India’s medical devices sector is poised to reach ₹6,36,463 crore by 2030, indicating significant job creation potential (AiMeD Industry Report).
This post will serve as your comprehensive guide to navigating the exciting and diverse career landscape of biomedical engineering in India in 2025. We will explore prominent industry sectors, examine technical specializations most relevant to the Indian context, outline essential education paths at top Indian institutions, discuss government initiatives boosting the field, and provide actionable pathways for aspiring students, professionals considering a career change, and current biomedical engineers seeking to advance their careers within the Indian healthcare ecosystem.
A biomedical engineer working with advanced holographic display technology at a leading research institution, representing the future of medical diagnostics.
Table of Contents
Indian Industry Sectors & Key Employers in 2025
The biomedical engineering careers in India presents a diverse ecosystem of sectors in 2025, each offering unique opportunities aligned with India’s healthcare priorities. From major corporate hospitals to innovative startups developing frugal technology solutions, biomedical engineers are becoming essential across the healthcare value chain. Understanding these sectors is crucial for making informed career choices in the Indian context.
The demand for biomedical engineers spans across leading hospital chains, rapidly growing medical device manufacturers, government and private research institutions, and the burgeoning healthtech startup ecosystem. Each environment offers different career trajectories shaped by India’s unique healthcare challenges and opportunities.
| Industry Sector | Key Focus Areas 2025 | Example Roles | Major Indian Employers | Est. Annual Salary Range (₹) |
|---|---|---|---|---|
| Hospitals & Healthcare Systems | Healthcare technology management, telemedicine infrastructure, data security, affordable medical equipment maintenance, biomedical waste management, equipment procurement for tier 2/3 cities. | Clinical Engineer, Biomedical Equipment Technician (BMET), Healthcare Technology Manager, Medical Device Integration Specialist. | Apollo Hospitals, Fortis Healthcare, Max Healthcare, Narayana Health, AIIMS network, Manipal Hospitals, Medanta. | ₹4,00,000 – ₹12,00,000 (AmbitionBox) |
| Medical Device Companies | Indigenous device development, affordable diagnostics, frugal innovation, point-of-care testing solutions, regulatory compliance (CDSCO, BIS), local manufacturing under PLI scheme, adaptation of global technologies for Indian market. | R&D Engineer, Regulatory Affairs Specialist, Quality Assurance Engineer, Manufacturing Engineer, Product Manager. | Trivitron Healthcare, Transasia Bio-Medicals, Skanray Technologies, Healthium Medtech, Perfint Healthcare, BPL Medical Technologies, Wipro GE Healthcare. | ₹6,00,000 – ₹15,00,000+ (Glassdoor) |
| Research Institutions & Academia | Biomaterials research, affordable healthcare technology, telemedicine solutions for rural areas, biomedical waste management, antimicrobial resistance research, non-invasive diagnostic tools, computational modeling. | Research Scientist, Faculty, Lab Manager, Grant Writer, Project Coordinator. | IISc Bangalore, SCTIMST Trivandrum, THSTI Faridabad, NIPER network, IITs with biomedical programs (IIT-B, IIT-D, IIT-M), CSIR-CMERI, AIIMS Biomedical Departments. | ₹5,00,000 – ₹14,00,000 (based on experience and institution) (PayScale) |
| Startups & Healthtech Firms | AI/ML for diagnostics, affordable point-of-care devices, remote patient monitoring, telemedicine innovations, wearable technology adapted for Indian conditions, healthcare data analytics, medical device prototyping. | Biomedical Product Lead, R&D Scientist, Co-founder, Innovation Officer, Process Development Engineer. | Agappe Diagnostics, Niramai Health Analytix, SigTuple, Forus Health, Biosense Technologies, Cyclops Medtech, Briota Technologies, Agatsa, Wellthy Therapeutics. | Highly variable, often includes equity. Base: ₹6,00,000 – ₹18,00,000+ (Inc42 DataLabs) |
| Government & Public Sector | Medical equipment standardization, public health technology implementation, regulatory oversight, healthcare infrastructure development, strategic health technology assessment. | Technical Officer, Biomedical Consultant, Regulatory Specialist, Healthcare Technology Assessment Officer. | CDSCO, National Health Mission, NHSRC, Department of Biotechnology, Central Drugs Standard Control Organisation, State Health Departments, NITI Aayog. | ₹5,00,000 – ₹12,00,000 (based on grade and experience) (7th Pay Commission) |
The growth drivers for these sectors in India are multifaceted. The government’s push for “Make in India” and “Atmanirbhar Bharat” initiatives has incentivized domestic medical device manufacturing. Simultaneously, India’s healthcare infrastructure development, including the Ayushman Bharat scheme providing healthcare coverage to over 500 million citizens, is driving demand for affordable and appropriate biomedical solutions. The COVID-19 pandemic has further accelerated digital health adoption and highlighted the need for self-reliance in critical medical equipment.

In-Demand Technical Specializations for Indian BMEs in 2025
The biomedical engineering landscape in India is evolving to address unique healthcare challenges while leveraging global technological advancements. By 2025, certain specializations will be particularly relevant in the Indian context, driven by the dual need for cutting-edge innovation and affordable healthcare solutions. These specializations represent areas where Indian biomedical engineers can make significant contributions to both domestic healthcare needs and global markets.
AI & Machine Learning in Indian Healthcare
Core Focus: AI and Machine Learning applications in Indian healthcare are uniquely positioned to address challenges of accessibility, affordability, and the shortage of specialists. Indian biomedical engineers in this specialization develop algorithms that can function effectively with limited computational resources and intermittent connectivity—essential for deployment in tier-2/3 cities and rural areas. They focus on creating AI tools for screening common conditions prevalent in India, such as tuberculosis detection from chest X-rays, diabetic retinopathy screening, and maternal health monitoring. These systems are often designed to be operated by healthcare workers with minimal technical training, maximizing their utility in resource-constrained settings (ICMR AI in Healthcare).
Key Technical Skills Needed (2025): Proficiency in Python with frameworks like TensorFlow and PyTorch remains essential, but with additional emphasis on model optimization for deployment on resource-constrained hardware. Skills in developing offline-capable applications that can sync data when connectivity is available are highly valued. Understanding of Indian health data standards and integration with Ayushman Bharat Digital Mission (ABDM) frameworks is increasingly important. Expertise in developing multilingual NLP models capable of processing medical information in Indian languages is becoming crucial for wider adoption.
Indian Market Applications: The horizon for AI/ML in Indian healthcare is expansive and includes AI-assisted tuberculosis screening programs deployable at primary health centers, mobile-based diagnostic tools for use by ASHA workers (community health activists), AI systems for monitoring maternal health in rural settings, and predictive analytics for managing chronic conditions like diabetes and hypertension that are becoming increasingly prevalent in India. Companies like Niramai have pioneered affordable AI-based breast cancer screening tools specifically designed for the Indian context.
Career Potential in India: The demand for biomedical engineers with AI/ML expertise is growing rapidly in India. Roles include AI Health Research Scientist at institutions like IIIT-Delhi’s Center for Artificial Intelligence, Machine Learning Engineer at healthtech startups like SigTuple or Niramai, Clinical Informatics Specialist at major hospital chains, and AI Product Manager positions at established healthcare companies adapting global technologies for Indian markets.
If you want to know more about AI in Medical Equipment click here.
Medical Robotics & Frugal Innovation
Core Focus: Medical robotics in the Indian context emphasizes affordability and appropriateness for local healthcare settings. Biomedical engineers in this specialization work on developing cost-effective robotic solutions for surgery, rehabilitation, and hospital automation. Rather than simply importing expensive robotic systems, the focus is on frugal innovation—creating systems that deliver maximum value at minimum cost. This includes developing modular surgical robotics platforms that can be manufactured locally, affordable exoskeletons for rehabilitation, and automation solutions for tasks like sample collection and medicine dispensing that address the healthcare workforce shortages common in India (DST National Robotics Roadmap).
Key Technical Skills Needed (2025): Proficiency in ROS (Robot Operating System) remains important, but with additional focus on developing systems that can function reliably in challenging environments (unstable power, varying temperature/humidity). Skills in reverse engineering and adapting existing technologies for local manufacturing are highly valued. Knowledge of India-specific regulatory requirements for robotic medical devices is essential. Experience in designing user interfaces suitable for operators with varying technical literacy levels is important for widespread adoption.
Indian Market Applications: Emerging applications include affordable surgical robotics systems developed by companies like SS Innovations, rehabilitation robots for district hospitals and specialized centers, telepresence robots enabling specialist consultations in remote areas, and automated systems for pharmaceutical compounding and dispensing. There is also growing interest in developing robotic solutions for elderly care as India’s demographic profile changes.
Career Potential in India: Career opportunities in this specialization are growing, with roles such as Robotics Engineer at companies like Perfint Healthcare (pioneers in robotic assisted procedures), Rehabilitation Technology Specialist at hospitals and therapy centers, R&D Engineer at indigenous medical device startups, and Automation Consultant for hospital systems. Research positions are available at institutions like IIT Madras’s Center for Industrial Consultancy and Sponsored Research, which focuses on affordable healthcare technologies.
Tissue Engineering & Regenerative Medicine
Core Focus: Tissue engineering in India focuses on developing culturally and economically appropriate solutions for the Indian population. Biomedical engineers in this field work on creating affordable biomaterials using locally available resources, developing tissue constructs suited to Indian genetic profiles, and advancing treatments for conditions prevalent in the Indian population. There is particular emphasis on developing cost-effective strategies for wound healing (especially for diabetic patients), bone regeneration therapies, and corneal tissue engineering to address the high incidence of corneal blindness in India (DBT India Tissue Engineering Program).
Key Technical Skills Needed (2025): Expertise in working with indigenous biomaterials and adapting protocols to local conditions is essential. Proficiency in developing and maintaining cell cultures under challenging infrastructure conditions (e.g., intermittent power) is valuable. Knowledge of Good Manufacturing Practices (GMP) adapted to Indian regulatory frameworks is critical for translational work. Understanding of scale-up processes that are feasible within Indian manufacturing capabilities is important for moving technologies from lab to market.
Indian Market Applications: Notable applications include affordable skin substitutes for burn victims and diabetic ulcer patients, bioengineered corneal tissue to address the significant backlog of corneal transplants needed in India, scaffolds for bone regeneration using materials derived from natural Indian resources, and low-cost bioreactors for cell expansion that can function reliably in typical Indian hospital settings.
Career Potential in India: Career paths include Research Scientist positions at institutions like the National Centre for Cell Science (NCCS) Pune or Institute for Stem Cell Science and Regenerative Medicine (inStem) Bangalore, Tissue Engineer at companies like Pandorum Technologies working on bioengineered tissues, and roles at hospitals developing translational regenerative medicine programs. Teaching positions at institutions offering specialized biomedical programs, such as BITS Pilani and Manipal Academy of Higher Education, are also growing.

A biomedical researcher meticulously working with a microscope and laboratory equipment, essential for advancements in tissue engineering and regenerative medicine
Affordable Medical Devices & Indigenous Innovation
Core Focus: This specialization centers on developing medical devices that are affordable, robust, and appropriate for Indian healthcare settings. Biomedical engineers in this field work on “Make in India” medical technology solutions that address local healthcare challenges while reducing dependence on imports. Key focus areas include point-of-care diagnostic devices suitable for primary health centers, patient monitoring systems designed to function in resource-limited settings, affordable imaging solutions, and innovative devices addressing India-specific disease patterns. The emphasis is on creating technologies that can function reliably despite infrastructure challenges like power fluctuations or limited technical support (ICMR-DHR Medical Device Policy).
Key Technical Skills Needed (2025): Proficiency in CAD/CAM software remains important, but with additional focus on designing for local manufacturing capabilities and supply chains. Skills in developing low-power electronics and energy-efficient systems are highly valued. Knowledge of India’s medical device regulations, including the new Medical Devices Rules and BIS certification requirements, is essential. Experience in frugal engineering principles—maximizing value while minimizing resources—is particularly important in the Indian context.
Indian Market Applications: Noteworthy applications include portable diagnostic devices for rural healthcare settings (like the work of Biosense Technologies), affordable alternatives to imported medical equipment (such as Skanray’s critical care equipment), innovative monitoring devices designed for hot and humid conditions, and specialized equipment addressing tropical diseases. There is also growing emphasis on developing assistive technologies tailored to Indian user needs and socioeconomic contexts.
Career Potential in India: Career opportunities include Medical Device Engineer positions at Indian manufacturers like Trivitron Healthcare or BPL Medical Technologies, R&D roles at multinational companies’ Indian innovation centers, Product Development positions at startups like Forus Health (developing eye care devices), and regulatory affairs specialists focusing on navigating India’s evolving medical device regulatory landscape. Opportunities also exist with government initiatives like the Biotechnology Industry Research Assistance Council (BIRAC), which supports indigenous medical technology development.
Essential Education & Skill Requirements in India
Building a successful career in biomedical engineering in India requires a strong educational foundation aligned with the country’s healthcare needs and technological landscape. The interdisciplinary nature of the field demands both technical expertise and a deep understanding of the unique challenges in Indian healthcare delivery.
Educational Pathways in India
India offers multiple academic routes to enter the biomedical engineering profession, with increasing specialization options becoming available at leading institutions across the country.
Degrees & Top Indian Institutions:
- Bachelor’s Degree (B.Tech/B.E. in Biomedical Engineering or related fields): This forms the foundation for most entry-level roles. Leading institutions offering undergraduate BME programs include:
- IIT Bombay – B.Tech in Biomedical Engineering
- IIT Delhi – B.Tech with specialization in Biomedical Engineering
- Manipal Institute of Technology – B.Tech in Biomedical Engineering
- VIT Vellore – B.Tech in Biomedical Engineering
- SRM University – B.Tech in Biomedical Engineering
- BITS Pilani – B.E. in Electronics & Instrumentation with Biomedical focus
- Master’s Degree (M.Tech/M.E. in Biomedical Engineering, often with specialization): Advanced degrees are increasingly important for research and specialized roles. Premier institutions include:
- IIT Bombay – M.Tech in Biomedical Engineering
- IIT Madras – M.Tech in Biotechnology with Biomedical focus
- IISc Bangalore – M.Tech in Bioengineering
- AIIMS New Delhi – M.Biotechnology with Medical Technology options
- CMC Vellore – M.Tech in Clinical Engineering
- SCTIMST Trivandrum – M.Tech in Clinical Engineering
- Doctoral Degree (Ph.D. in Biomedical Engineering or related areas): Required for high-level research and academic positions. Leading institutions include:
- IIT Bombay – Center for Biomedical Engineering
- IIT Delhi – Center for Biomedical Engineering
- IISc Bangalore – Department of Biosciences and Bioengineering
- SCTIMST Trivandrum – Biomedical Technology Wing
- THSTI Faridabad – Interdisciplinary Biomedical Research programs
India-Specific Certifications & Professional Recognition:
- Association of Biomedical Engineers of India (ABEI) Professional Certification: Increasingly recognized for clinical engineering roles in hospitals, this certification validates knowledge of medical equipment management in Indian healthcare settings.
- Quality Council of India (QCI) – Medical Devices Certification: Important for roles in regulatory affairs and quality management in the growing Indian medical devices industry.
- Indian Society for Clinical Engineering (ISCE) Certifications: Focused on healthcare technology management in Indian hospital environments.
- Electronics Sector Skills Council of India (ESSCI) – Biomedical Equipment Technician Certification: Relevant for technical support and equipment maintenance roles in smaller hospitals and clinics.
- Regulatory Skills Certification by AERB: For biomedical engineers working with radiological equipment in India.
Foreign Certifications Valued in India: International certifications also hold value, particularly in multinational companies operating in India or for professionals seeking global mobility:
- Certified Clinical Engineer (CCE): Recognized by leading corporate hospital chains in India.
- Project Management Professional (PMP): Valued for management roles in medical device companies and large healthcare projects.
- International Compliance Certifications: Such as those related to ISO 13485 or FDA regulations, important for export-oriented medical device manufacturers in India.
Key Skill Sets for Indian BMEs in 2025
Beyond formal education, biomedical engineers in India need specific technical and soft skills relevant to the local healthcare ecosystem.
Technical Skills Matrix for India:
| Skill Category | India-Specific Examples for 2025 |
|---|---|
| Programming & Software Proficiency | Python (essential for AI/ML applications in Indian healthcare), MATLAB (simulation, data analysis), C/C++ (embedded systems for resource-constrained devices), CAD software (for local manufacturing design), knowledge of Hospital Information Systems commonly used in India (e.g., e-Hospital, HMS by C-DAC), familiarity with ABDM (Ayushman Bharat Digital Mission) standards and APIs. |
| Frugal Innovation & Appropriate Technology | Design for resource-constrained settings, low-power electronics for areas with unreliable electricity, knowledge of locally available materials and manufacturing capabilities, ability to develop solutions that function in diverse environmental conditions (heat, humidity, dust), expertise in creating maintenance-friendly designs for settings with limited technical support. |
| Clinical & Healthcare System Knowledge | Understanding of India’s multi-tiered healthcare system (from primary health centers to tertiary care), knowledge of disease patterns and healthcare priorities specific to India, familiarity with Ayushman Bharat and other national health programs, awareness of healthcare delivery challenges in urban vs. rural settings. |
| Regulatory & Standards Knowledge | Familiarity with Medical Devices Rules 2017 (updated versions), understanding of CDSCO requirements and approval processes, knowledge of BIS (Bureau of Indian Standards) certification for medical devices, awareness of India’s evolving regulatory landscape including the new Medical Devices (Amendment) Rules, understanding of export compliance for medical devices manufactured in India. |
| Local Manufacturing & Supply Chain | Knowledge of Indian manufacturing capabilities and limitations, understanding of local component sourcing, expertise in design for manufacturability within Indian industrial ecosystem, familiarity with Production Linked Incentive (PLI) scheme requirements, ability to adapt global designs for local production. |
Essential Soft Skills for Indian Context:
- Cross-cultural Communication: Ability to communicate technical concepts effectively across diverse language backgrounds and varying levels of technical literacy, particularly important when working with healthcare professionals and patients from different socioeconomic backgrounds.
- Adaptability & Resourcefulness: Capacity to work effectively despite infrastructure limitations and to develop creative solutions with available resources—a critical skill in many Indian healthcare settings.
- Cost-conscious Innovation: Ability to balance technological sophistication with affordability, developing solutions that are financially accessible to Indian healthcare providers and patients.
- Collaborative Problem-solving: Skill in working with multidisciplinary teams spanning medicine, engineering, public health, and business—essential in addressing complex healthcare challenges in the Indian context.
- Healthcare System Navigation: Understanding of how to effectively navigate India’s complex healthcare ecosystem, including public and private sectors, to successfully implement new technologies.
- Social Impact Orientation: Awareness of how biomedical innovations can address healthcare disparities and improve access for underserved populations, particularly relevant in the Indian context.
- Entrepreneurial Mindset: Ability to identify opportunities and develop viable business models for biomedical innovations within the Indian market context.
- Patient-centered Design Thinking: Skill in developing solutions that consider the specific needs, preferences, and constraints of Indian patients and healthcare providers.
Government Initiatives Boosting Biomedical Engineering in India
India’s biomedical engineering ecosystem is receiving unprecedented support through various government initiatives aimed at strengthening healthcare technology innovation, manufacturing, and implementation. These programs create significant opportunities for biomedical engineers across research, industry, and healthcare delivery.
Key National Initiatives:
- Production Linked Incentive (PLI) Scheme for Medical Devices: Launched in 2020 and expanded thereafter, this scheme offers financial incentives to boost domestic manufacturing of medical devices. With an outlay of ₹3,420 crore, it incentivizes production in priority segments including cancer care/radiotherapy equipment, radiology & imaging devices, anesthetics & cardio-respiratory equipment, and implants. For biomedical engineers, this creates numerous jobs in R&D, manufacturing, quality assurance, and regulatory affairs within domestic medical device companies (Ministry of Chemicals & Fertilizers).
- Medical Devices Parks: The government is establishing dedicated medical devices parks in states including Andhra Pradesh, Telangana, Tamil Nadu, and Kerala. These parks provide shared infrastructure, testing facilities, and regulatory support to medical device manufacturers. They serve as innovation clusters creating opportunities for biomedical engineers to work on indigenous device development and manufacturing optimization.
- National Biopharma Mission/Innovate in India (i3): This World Bank-supported mission under the Department of Biotechnology promotes industry-academia collaboration for developing affordable biopharmaceuticals and medical devices. With a budget of $250 million, it supports projects addressing India’s public health priorities. For biomedical engineers, the mission funds research positions and creates industry opportunities in biopharma and medical technology innovation (BIRAC).
- Biotechnology Industry Research Assistance Council (BIRAC): This public sector enterprise under DBT supports biotech and medical technology startups through funding, mentorship, and infrastructure access. Programs like BIG (Biotechnology Ignition Grant), PACE, and SPARSH specifically support healthcare technology innovations. For biomedical engineers, BIRAC creates entrepreneurship opportunities and funding for translational research.
- Ayushman Bharat Digital Mission (ABDM): This flagship initiative aims to develop an integrated digital health infrastructure for India, including electronic health records, telemedicine, and health analytics. The mission is creating demand for biomedical engineers with expertise in healthcare informatics, medical data standards, and interoperability solutions (National Health Authority).
- Department of Science & Technology (DST) Healthcare Technology Programs: DST funds research and innovation in healthcare technologies through initiatives like the Technology Development Board and SEED (Science for Equity, Empowerment & Development). These programs support projects developing affordable diagnostics, medical devices, and assistive technologies suited to Indian needs.
- National Medical Devices Promotion Council: Established under the Department for Promotion of Industry & Internal Trade (DPIIT), this council promotes domestic manufacturing, exports, and quality standards for medical devices. Its activities create opportunities for biomedical engineers in policy implementation, standards development, and industry growth initiatives.
Recent Policy Developments (2023-2025):
- Medical Devices (Amendment) Rules: These updated regulations strengthen quality standards and regulatory oversight for medical devices manufactured or sold in India. They create increased demand for biomedical engineers with regulatory expertise and knowledge of quality management systems.
- Artificial Intelligence in Healthcare Policy Framework: Developed by NITI Aayog in collaboration with health ministry, this framework guides the ethical development and implementation of AI solutions in Indian healthcare. It opens opportunities for biomedical engineers specializing in AI/ML for healthcare applications.
- National Digital Health Blueprint: This comprehensive framework outlines India’s strategy for digital health transformation, covering areas from telemedicine to health analytics. It creates roles for biomedical engineers in developing interoperable medical devices, healthcare IT systems, and digital health solutions.
- Make in India Medical Devices 2.0: This updated initiative specifically targets reducing import dependence for medical devices through enhanced domestic manufacturing capabilities. It includes targeted interventions for high-value medical equipment segments, creating opportunities for biomedical engineers in product development and manufacturing.
Impact on BME Career Opportunities
These government initiatives are transforming the biomedical engineering landscape in India through multiple pathways:
- R&D Funding: Increased research grants from DBT, DST, and other agencies are creating positions for biomedical engineers in universities, research institutions, and industry R&D centers.
- Startup Ecosystem: Support for healthcare startups through BIRAC, Startup India, and Atal Innovation Mission is creating entrepreneurial opportunities and positions in healthtech ventures.
- Manufacturing Growth: The PLI scheme and medical devices parks are expanding domestic medical device manufacturing, creating jobs in design, production, quality control, and regulatory compliance.
- Public Health Implementation: National health programs need biomedical engineers for technology assessment, procurement, maintenance, and training across the public healthcare system.
- Standards & Regulation: The evolving regulatory landscape has increased demand for biomedical engineers with expertise in quality systems and regulatory affairs.
For biomedical engineers, tracking these government initiatives and aligning skills with national priorities can open pathways to impactful and rewarding careers contributing to India’s healthcare transformation.
Practical Advice & Actionable Steps for Indian BMEs
Navigating a successful career in biomedical engineering in India requires strategic planning and action tailored to the unique opportunities and challenges of the Indian healthcare ecosystem. Here’s practical advice for different career stages, specifically contextualized for the Indian biomedical engineering landscape.
For Students (Undergraduate/Graduate) in India:
- Action: Secure Internships at Indian Healthcare Technology Leaders
How-to: Target internships at both established players and innovative startups in the Indian medical technology ecosystem. Apply to structured internship programs at companies like Trivitron Healthcare, Wipro GE Healthcare, and Transasia Bio-Medicals. For research experience, explore summer fellowship programs at premier institutions like IISc Bangalore’s Centre for BioSystems Science and Engineering, SCTIMST Trivandrum, or the CSIR-Central Scientific Instruments Organisation (CSIO). Utilize platforms like Internshala and the Indian Biomedical Skills Consortium for biomedical-specific internships. Consider clinical immersion internships at hospitals like AIIMS, Manipal Hospitals, or Apollo Hospitals to understand healthcare delivery challenges firsthand. - Action: Build Skills Aligned with India’s Healthcare Technology Needs
How-to: Supplement your academic education with practical skills addressing Indian healthcare challenges. Take courses on frugal engineering and appropriate technology design through platforms like NPTEL, which offers specialized biomedical engineering modules. Participate in healthcare hackathons organized by organizations like Healthcare Federation of India (NATHEALTH) or the Biodesign Innovation Fellowships at AIIMS/IIT Delhi. Join student chapters of professional organizations like the Indian Society for Clinical Engineering (ISCE) or Biomedical Engineering Society of India for networking and skill development. Learn about India’s medical device regulations through workshops conducted by organizations like the Association of Indian Medical Device Industry (AiMeD). - Action: Connect with the Indian BME Professional Community
How-to: Build strong professional connections within India’s biomedical engineering ecosystem. Attend industry conferences like the Medical Fair India, India Med Expo, or BioAsia. Join professional platforms like the Association of Biomedical Engineers of India (ABEI) as a student member to connect with practicing professionals. Follow and engage with organizations like BIRAC, MedTech Connect, and the Medical Technology Association of India on LinkedIn and at events. Participate in government-sponsored initiatives like the Smart India Hackathon’s healthcare challenges or the Biotechnology Ignition Grant’s student innovation programs.
For Career Changers in India:
- Action: Leverage Your Existing Skills for India’s BME Sector
How-to: Identify how your current expertise maps to needs in India’s biomedical engineering landscape. If you have IT background, explore healthtech companies like Practo, 1mg, or Netmeds that combine healthcare and technology. Engineers from other disciplines can transition into medical device roles at companies like Skanray Technologies or BPL Medical, which value strong engineering fundamentals. Professionals with management experience should explore roles in healthcare technology management at hospital chains or in business development at medical device companies. Pharmaceutical professionals can transition to regulatory affairs roles specific to medical devices, leveraging their understanding of India’s healthcare regulatory landscape. - Action: Acquire India-Specific BME Credentials
How-to: Pursue targeted education that bridges your background with biomedical engineering needs in India. Consider specialized post-graduate diplomas in areas like Healthcare Technology Management from institutions like IIHMR or BITS Pilani’s Work Integrated Learning Programs (WILP) in Healthcare Engineering, which can be completed while working. Explore Clinical Engineering certificate programs offered by institutions like SCTIMST Trivandrum or AIIMS. Attend regulatory workshops offered by organizations like Quality Council of India on the Medical Devices Rules. Complete short-term courses in healthcare-specific applications of your existing skills, such as healthcare data analytics or medical device quality management systems, available through platforms like Swayam or from healthcare industry associations. - Action: Network Strategically within India’s Healthcare Technology Ecosystem
How-to: Build connections specifically within India’s biomedical and healthtech community. Attend industry events like the Confederation of Indian Industry (CII) Healthcare Conclave or Medical Electronics Forum of ELCINA that bring together professionals from various backgrounds working in healthcare technology. Join WhatsApp groups and LinkedIn communities like “Medical Devices India” or “Healthcare Technology Management India” where professionals share opportunities and insights. Connect with incubators focusing on healthcare, such as C-CAMP in Bangalore, IKP Knowledge Park in Hyderabad, or the AMTZ MedTech Incubation Center in Visakhapatnam, which often host networking events and workshops.
For Current Biomedical Engineers in India (Seeking Advancement):
- Action: Specialize in High-Growth Areas for the Indian Market
How-to: Develop expertise in specializations particularly relevant to India’s healthcare needs. Consider advanced certification in AI for healthcare through programs like the AI in Healthcare course by NASSCOM or specialized workshops at IIT Delhi’s School of Artificial Intelligence. For those interested in regulatory pathways, pursue credentials in Indian medical device regulations through organizations like Quality Council of India or Regulatory Affairs Professionals Society (RAPS) India Chapter. Develop expertise in Ayushman Bharat Digital Mission standards and interoperability frameworks through programs offered by the National Health Authority. Consider advanced degrees with industry collaboration, such as the Healthcare Technology Innovation Centre’s programs at IIT Madras or the biodesign programs at IIT Delhi and AIIMS. - Action: Build Leadership Capabilities Contextual to Indian Healthcare
How-to: Develop management skills specifically relevant to India’s healthcare technology sector. Pursue healthcare management education through institutions like IIHMR or IIM healthcare management programs to complement your technical expertise. Take on cross-functional projects that give you exposure to multiple aspects of healthcare technology implementation in Indian settings. Participate in industry associations like the Medical Technology Association of India (MTaI) or NATHEALTH’s technology committees to gain broader industry perspective. Consider publishing in Indian journals like the Indian Journal of Medical Research or Journal of Medical Devices (India) to establish thought leadership. Mentor engineering students through programs like the Biomedical Engineering Society of India’s mentorship initiatives. - Action: Align with Government Initiatives and Public Health Priorities
How-to: Position your career to leverage India’s healthcare policy priorities. Stay informed about government initiatives through regular monitoring of announcements from ministries like Health, Science & Technology, and Electronics & IT. Participate in standards committees developing Indian standards for medical devices through Bureau of Indian Standards (BIS). Apply for funding through government schemes like BIRAC’s BIG grants or DST healthcare technology programs if you have innovative ideas. Consider roles in public sector organizations implementing healthcare technology initiatives, such as C-DAC’s healthcare informatics group, National Health Authority’s digital health teams, or state health departments’ technology divisions. Explore opportunities with government-supported medical technology parks being established across India.
Statistical Deep Dive: The Indian BME Career Landscape (2025 Outlook)
Understanding the quantitative aspects of the biomedical engineering career landscape in India is essential for making informed career decisions. This section provides data on salary ranges, job growth projections, and employment distribution specific to the Indian context.
Salary Insights (Indian Data)
Biomedical engineering offers competitive compensation in India, though with significant variation based on experience, specialization, and type of employer.
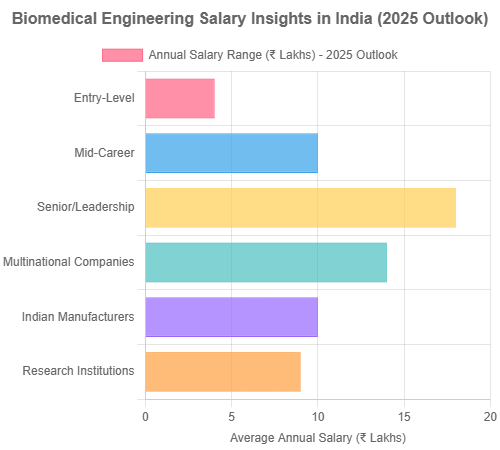
- Overall Median Annual Salary: Biomedical engineers in India earn a median annual salary of approximately ₹6,00,000 to ₹8,00,000, with significant variations based on experience, qualifications, and employer type (AmbitionBox).
- Salary Range: Entry-level positions typically start around ₹3,00,000 to ₹5,00,000 annually, while experienced professionals with 10+ years of experience can earn ₹15,00,000 to ₹25,00,000+ annually, particularly in specialized roles or leadership positions.
- By Experience Level (Illustrative):
- Entry-Level (0-2 years): ₹3,00,000 – ₹5,00,000Mid-Career (5-10 years): ₹8,00,000 – ₹15,00,000Senior/Lead Roles (10+ years): ₹15,00,000 – ₹25,00,000+
- By Sector in India: Salaries vary significantly across sectors:
- Multinational Medical Device Companies: Often offer the highest compensation (₹8,00,000 – ₹20,00,000+)
- Indian Medical Device Manufacturers: Generally offer moderate salaries (₹6,00,000 – ₹15,00,000)
- Hospitals: Clinical engineering roles typically range from ₹4,00,000 – ₹12,00,000
- Research Institutions: Generally offer ₹5,00,000 – ₹14,00,000 depending on qualification and position
- Startups: Often provide lower base salaries (₹4,00,000 – ₹10,00,000) but may include equity
- By Specialization: AI/ML specialists in healthcare and regulatory affairs professionals with medical device expertise often command premium salaries, sometimes 30-40% higher than general biomedical engineering roles.
Those entering Biomedical Engineering careers in India can expect promising remuneration and career progression. Entry-level positions in medical device manufacturing and hospital engineering typically offer ₹3–5 LPA, while mid‑level roles in R&D, AI healthcare, or regulatory affairs earn ₹6–12 LPA, with senior positions reaching ₹15–25 LPA annually. With increasing investment in MedTech innovation hubs—such as IIT Bombay’s BETiC—and government‑backed incentives, Biomedical Engineering careers in India are becoming more lucrative and impactful.
Job Growth Forecasts (Indian Data)
The biomedical engineering field in India is experiencing robust growth, driven by government initiatives, increasing healthcare spending, and the push for domestic medical device manufacturing.
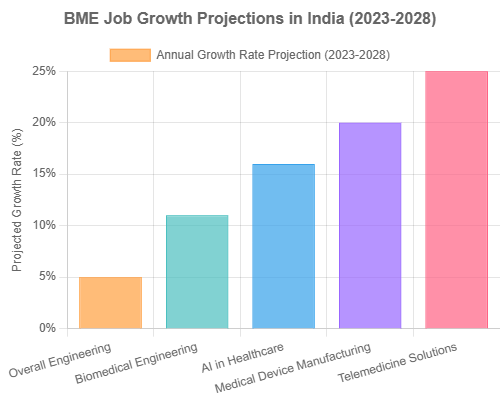
- Overall BME Job Growth Projection (2023-2028): Employment in biomedical engineering in India is projected to grow at 10-12% annually between 2023 and 2028, significantly outpacing many other engineering disciplines (Naukri Career Blog).
- Medical Device Industry Growth: India’s medical devices industry is expected to grow to $50 billion by 2025, expanding at a CAGR of approximately 15%. This represents a significant increase from approximately $11 billion in 2020, indicating substantial job creation potential (IBEF Medical Devices Industry Report).
- Geographic Distribution: Major employment hubs for biomedical engineers in India include:
- Bangalore (Karnataka): Known for healthtech startups and R&D centersHyderabad (Telangana): Home to medical device manufacturing and the AMTZMumbai & Pune (Maharashtra): Strong in pharmaceutical and medical device industriesDelhi NCR: Houses regulatory bodies and major hospitalsChennai (Tamil Nadu): Developing as a medical devices manufacturing hub
- Fastest Growing Areas in India: Areas showing particularly strong growth include:
- AI/ML applications for Indian healthcare challenges (15-18% growth)
- Domestic manufacturing of medical devices (20%+ growth due to PLI scheme)
- Telemedicine and remote monitoring solutions (25%+ growth accelerated by COVID-19)
- Healthcare informatics aligned with Ayushman Bharat Digital Mission (15%+ growth)
- Regulatory affairs for medical devices (12-15% growth due to evolving regulations)manufacturing to R&D and healthcare IT.
India’s healthcare and MedTech ecosystem is booming, creating exciting Biomedical Engineering careers in India across hospitals, research institutions, start‑ups, and regulatory sectors. As per recent industry outlooks, the rising demand for imaging systems, wearable diagnostics, AI‑enabled devices, and regenerative medicine is driving a surge in job roles for biomedical engineers. This upward trajectory signals that Biomedical Engineering careers in India are not only expanding in number but also diversifying in specialization—ranging from clinical engineering and device manufacturing to R&D and healthcare IT.
Employment Distribution in India
Biomedical engineers in India work across diverse sectors, with a distribution pattern reflecting the country’s healthcare technology ecosystem.
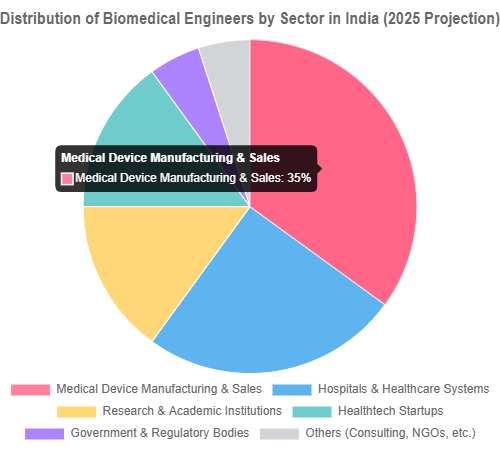
- Sector Distribution (2025 Projection):
- Medical Device Manufacturing & Sales: 35%
- Hospitals & Healthcare Systems: 25%
- Research & Academic Institutions: 15%
- Healthtech Startups: 15%
- Government & Regulatory Bodies: 5%
- Others (Consulting, NGOs, etc.): 5%
- Medical Device Manufacturing & Sales: 35%
- Employment Trends: India’s biomedical engineering employment landscape is undergoing several important shifts:
- Increasing roles in domestic manufacturing due to “Make in India” initiatives and PLI schemes
- Growing demand for regulatory specialists as India’s medical device regulatory framework evolves
- Rising opportunities in healthtech startups backed by significant venture capital investment
- Emerging roles in digital health tied to the Ayushman Bharat Digital Mission
- New positions in medical device testing and certification as quality control requirements strengthen
- Increasing roles in domestic manufacturing due to “Make in India” initiatives and PLI schemes
Key Takeaway: The data strongly highlights the rapid expansion and evolving nature of Biomedical Engineering careers in India. Fueled by government initiatives supporting domestic manufacturing, digital health advancements, and medical technology innovation, the field offers substantial growth potential. Biomedical engineers who align their skills with India’s national priorities—such as affordable healthcare technology, indigenous product development, and regulatory compliance—are well-positioned to thrive in this dynamic landscape.
Indian Biomedical Engineering in the Global Context
India’s biomedical engineering sector is increasingly integrated with the global healthcare technology ecosystem, creating unique opportunities at this intersection. This integration manifests through multiple pathways, positioning Indian biomedical engineers to contribute to and benefit from global innovations while addressing local healthcare challenges.
India as a Global R&D Hub
India has emerged as a significant research and development hub for biomedical engineering, with multinational companies establishing dedicated innovation centers in the country:
- Global Innovation Centers: Companies like GE Healthcare, Philips, Siemens Healthineers, and Boston Scientific have established major R&D facilities in India, particularly in Bangalore, Hyderabad, and Gurgaon. These centers employ thousands of Indian biomedical engineers working on technologies for both global and local markets.
- Frugal Innovation Export: Technologies developed in India to address local constraints are increasingly being adopted in other emerging markets and even advanced economies. Innovations like portable ECG devices, low-cost infant warmers, and smartphone-based diagnostic tools developed in India are finding global applications.
- Collaborative Research: Indian research institutions like IISc, IITs, and SCTIMST maintain active international research collaborations with institutions like Harvard, Stanford, MIT, and Imperial College London, creating pathways for Indian biomedical engineers to engage with global research networks.
Medical Device Manufacturing: From Import Dependence to Export Potential
India’s position in the global medical device value chain is evolving rapidly:
- Current Import Dependence: India currently imports approximately 80% of its medical devices, representing a significant trade deficit. High-end imaging equipment, implants, and sophisticated diagnostic devices remain heavily imported (IBEF Medical Devices Report).
- Emerging Export Capabilities: Several Indian companies have successfully entered global markets with products like ECG machines, patient monitors, and surgical instruments. The global market for affordable medical technologies provides significant export opportunities for Indian manufacturers.
- Contract Manufacturing Growth: India is emerging as a contract manufacturing destination for global medical device companies, particularly for products requiring precision engineering at competitive costs. This creates opportunities for biomedical engineers in quality assurance, manufacturing process optimization, and technology transfer.
- Supply Chain Integration: Indian medical device manufacturers are increasingly integrated into global supply chains, either as component suppliers or as value-adding partners creating customized solutions for various markets.
Digital Health & Healthtech: Cross-Border Opportunities
Digital health represents a domain where Indian biomedical engineers are making significant global contributions:
- Telemedicine Expertise: India’s experience in delivering healthcare services remotely to underserved areas has created valuable expertise that is now being exported to other countries with similar challenges.
- Health Data Analytics: Indian biomedical engineers with expertise in health data analytics are contributing to global projects through both Indian companies and multinational organizations.
- Global Startup Ecosystem: Indian healthtech startups are increasingly raising funding from international investors and expanding to global markets. Companies like Tricog Health, SigTuple, and Niramai have achieved international recognition.
- Remote Work Opportunities: Digital health platforms enable Indian biomedical engineers to work remotely for global companies, creating new employment pathways beyond traditional local opportunities.
Regulatory Harmonization & Quality Standards
India’s regulatory landscape for biomedical engineering is evolving in alignment with global standards:
- Regulatory Convergence: India’s medical device regulations are increasingly harmonized with global standards, particularly through adoption of principles from International Medical Device Regulators Forum (IMDRF).
- Quality Management Systems: Indian manufacturers are adopting international quality standards like ISO 13485, FDA quality system regulations, and EU MDR requirements to enter global markets.
- Global Career Mobility: Expertise in international regulatory frameworks creates career opportunities for Indian biomedical engineers with multinational companies and in export-oriented businesses.
- Certification Bodies: The emergence of internationally accredited testing and certification facilities in India, particularly at the Andhra Pradesh MedTech Zone (AMTZ), is facilitating greater integration with global regulatory systems.
Talent Flow & Knowledge Exchange
The movement of people and knowledge between India and global markets creates dynamic opportunities:
- Reverse Brain Gain: Increasing numbers of Indian biomedical engineers with international experience are returning to India, bringing global expertise to local challenges. Programs like the Ramalingaswami Re-entry Fellowship specifically support this transition.
- Educational Exchanges: Indian institutions are developing exchange programs and joint degrees with international universities, providing Indian students with global exposure while maintaining local relevance.
- Virtual Collaboration: Remote work and digital collaboration tools enable Indian biomedical engineers to participate in global teams and projects without relocation.
- Knowledge Transfer Initiatives: Organizations like AIMED (Association of Indian Medical Device Industry) facilitate knowledge exchange between Indian and international stakeholders through conferences, training programs, and joint initiatives.
Strategic Advantages for Indian Biomedical Engineers in the Global Context
Indian biomedical engineers possess several unique advantages in the global marketplace:
- Bridge Between Markets: Understanding both advanced technological capabilities and emerging market constraints positions Indian BMEs to develop solutions with global relevance.
- Frugal Engineering Expertise: The ability to optimize designs for cost-effectiveness and resource efficiency is increasingly valued even in developed markets focused on healthcare cost containment.
- English Language Proficiency: Strong communication skills facilitate collaboration with global teams and international clients.
- Experience with Scale: Working in the Indian healthcare system provides experience with high-volume, diverse patient populations that is valuable for developing robust, widely applicable solutions.
- Cultural Adaptability: Familiarity with navigating diverse cultural contexts within India translates to effectiveness in international collaborations.
For Indian biomedical engineers, strategic engagement with global opportunities while addressing local needs creates a powerful pathway to impactful and rewarding careers that transcend geographical boundaries.
The Future is Engineered by You: Embark on Your BME Journey in India
The biomedical engineering landscape in India in 2025 reflects a powerful convergence of opportunity, national importance, and potential for meaningful impact. As highlighted throughout this guide, the country’s specific healthcare needs and advancing tech infrastructure are shaping distinct and rewarding Biomedical Engineering careers in India, allowing professionals to drive both domestic healthcare transformation and global medical innovation.
The robust growth projections for India’s medical device industry—reaching $50 billion by 2025—coupled with government initiatives like Production Linked Incentives, Medical Device Parks, and the Ayushman Bharat Digital Mission clearly signal the strategic importance of this field. For biomedical engineers, this translates not just to job security but to the profound opportunity to shape a sector considered vital to national healthcare sovereignty.
What makes biomedical engineering in India particularly compelling is its potential for transformative impact. In a country where healthcare accessibility remains challenging for millions, innovative engineering solutions can bridge critical gaps. Whether developing affordable diagnostic tools deployable in primary health centers, creating robust medical equipment for resource-constrained settings, or pioneering digital health solutions that extend specialist care to remote areas, Indian biomedical engineers have the opportunity to directly improve countless lives.
The diversity of pathways available—from research institutions and hospitals to manufacturers and startups—allows professionals to align their careers with their passions and strengths. The growing ecosystem of support, including government funding, incubators, and industry associations, provides resources previously unavailable to innovators in this space.
Whether you’re a student exploring academic options, a professional seeking a career shift, or an experienced engineer charting your next move, the outlook is clear: Biomedical Engineering careers in India offer a rare blend of intellectual rigor, financial growth, and societal impact. By cultivating in-demand skills, forging strong industry connections, and aligning with both national priorities and global trends, you can become a driving force in India’s healthcare technology evolution.
The trajectory of healthcare in India is being defined now—and it’s the innovative minds behind Biomedical Engineering careers in India who will shape it. That future of accessible, advanced, and locally-driven healthcare is yours to engineer.
What excites you most about Biomedical Engineering careers in India**? Are you passionate about creating cost-effective medical technologies, contributing to breakthrough research, or enhancing healthcare delivery systems nationwide? We’d love to hear your thoughts—drop your questions and insights in the comments below!**
To stay updated on the fast-evolving landscape of Biomedical Engineering careers in India, subscribe to our blog. Upcoming posts will explore funding avenues for healthtech startups, spotlight top Indian BME academic programs, and unravel the regulatory process for medical device approval in India.
