After nearly two decades in biomedical engineering, I’ve seen the industry transform—especially in vibrant markets like India. Propelled by government initiatives such as Make in India and the Ayushman Bharat scheme, the Indian MedTech sector is booming. Today, the lines between mechanical, electrical, and biological engineering are increasingly blurred by AI in diagnostics, wearable health technologies, and 3D-printed implants [careerplanneredufair.com]. In this rapidly evolving landscape, a B.Tech degree is just the starting point. To truly thrive and lead, professionals must demonstrate specialized expertise, and this is where Biomedical Engineer Certifications play a crucial role in shaping long-term career growth and industry relevance.
Pursuing the right biomedical engineer certifications is no longer a ‘nice-to-have’—it’s a critical career accelerator for aspiring professionals in India and its neighboring countries. It signals to employers at home and abroad that you are committed to excellence, fluent in global and local standards, and possess skills that go beyond the core curriculum. With the Indian medical device market poised for significant growth, standing out is paramount.
This guide is my ‘from the trenches’ perspective on the certifications that will deliver the highest ROI for your career in 2026. Whether you’re a student in India, Nepal, Bangladesh, or Sri Lanka mapping out your future, an early-career engineer eager to advance, or a seasoned professional eyeing a leadership role in a top MedTech hub like Bengaluru or Hyderabad, this article will provide an actionable roadmap. We will explore certifications in:
- Quality Management & Compliance (ISO 13485, CDSCO)
- Regulatory Affairs
- Project Management
- Technical Specialization
Table of Contents
Why Certifications Matter More Than Ever in 2026
In a competitive job market, a biomedical engineer certifications is a powerful differentiator. It’s evidence-based proof of your skills. But the benefits go far beyond just looking good on a resume. Here’s the real-world impact I’ve witnessed, especially within the Indian context.
Significant Salary and Career Advancement
Let’s talk numbers. In India, salaries for biomedical engineers vary widely based on experience and specialization. An entry-level engineer might start with a salary between ₹2.5 LPA and ₹5 LPA. However, with experience and the right credentials, this can quickly rise to ₹6-12 LPA for mid-level roles and ₹13-25 LPA for senior positions [careerplanneredufair.com]. Adding a Project Management Professional (PMP)® certification can significantly increase earning potential, opening doors to management roles in multinational corporations with R&D centers in India [netcomlearning.com].
Demonstrating Specialized Expertise
Biomedical engineering is incredibly broad. biomedical engineer certifications allow you to formally validate your expertise in a high-demand niche. A Certified Quality Engineer (CQE) credential, for instance, proves your mastery of quality control principles vital for medical device manufacturing, a sector the Indian government is heavily promoting.
Building Credibility and Navigating Regulations
The medical device industry is one of the most regulated in the world. For companies in India, compliance is twofold: meeting the standards of the Central Drugs Standard Control Organisation (CDSCO) for the domestic market and adhering to international standards like ISO 13485, CE (for Europe), and FDA (for the US) for exports. Having team members who are certified or knowledgeable in these frameworks is a massive asset. It builds trust with regulators and global partners by demonstrating a foundational commitment to safety and quality [iso.org].
Who This Guide Is For
This guide is tailored to empower professionals across India and South Asia:
- Current B.Tech/B.E. Students: Gain a competitive edge before you graduate from an Indian or regional university.
- Entry-Level Engineers (0-3 years): Accelerate your growth in companies like Siemens Healthineers, GE Healthcare, or numerous Indian startups.
- Mid-Career Professionals (3-10 years): Target leadership roles like QA Manager, Clinical Engineer, or R&D Team Lead.
- Biomedical Equipment Technicians (BMETs): Bridge the gap from technician to engineer with advanced compliance and technical certifications.
- Career Switchers & Professionals from SAARC Nations: Validate your skills and demonstrate your commitment to the healthcare technology sector, whether you’re in Pakistan, Nepal, Sri Lanka, or Bangladesh.
Top Biomedical Engineer Certifications for 2026
Here is my breakdown of the most impactful Biomedical Engineer Certifications, categorized by core competency and adapted for the Indian and South Asian job markets.
Category 1: Quality Management & Compliance
Quality is the bedrock of medical technology. These certifications prove you speak the language of quality, both locally and globally.
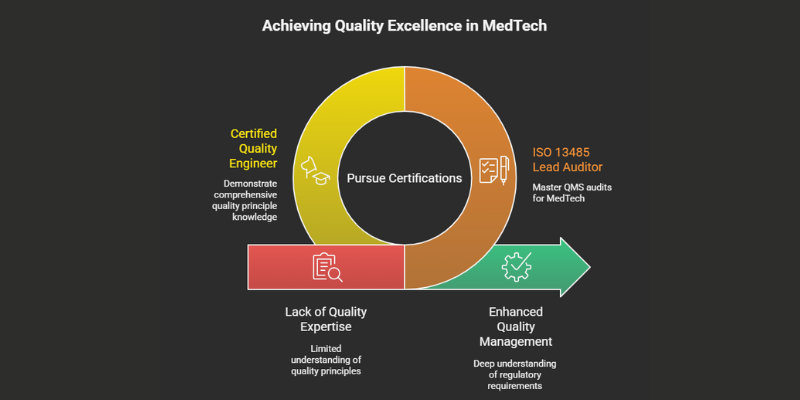
1. ISO 13485 Lead Auditor
- What It Is: ISO 13485 is the international standard for quality management systems (QMS) in the medical device industry, which is also a key compliance requirement under India’s Medical Device Rules. While companies get certified, individuals can become Certified Lead Auditors, qualifying them to conduct QMS audits.
- Who It’s For: Mid-career professionals, QA/QC engineers, manufacturing engineers, and anyone aspiring to a quality management role in an Indian or multinational MedTech company.
- Why It’s Valuable: It demonstrates a deep understanding of the regulatory requirements for designing and manufacturing medical devices for both domestic and international markets. This is a core competency for any serious MedTech company in India [qualio.com].
- Path to Certification: Typically involves a 4-day intensive training course from a certified provider.
- Cost: Approximately ₹80,000 – ₹1,50,000, depending on the training provider.
- Prerequisites: None are strictly required for the course, but prior knowledge of quality management systems is highly recommended.
2. Certified Quality Engineer (CQE)
- What It Is: A prestigious certification from the American Society for Quality (ASQ) that demonstrates a comprehensive understanding of quality principles.
- Who It’s For: Entry-level to mid-career engineers in R&D, manufacturing, or supplier quality in the numerous manufacturing hubs across India.
- Why It’s Valuable: The CQE Body of Knowledge is exhaustive, covering everything from statistical process control to risk assessment. It’s a powerful, globally recognized credential that signals a commitment to data-driven quality excellence.
- Path to Certification: Requires eight years of on-the-job experience, but a B.Tech/B.E. degree waives four of those years [asq.org]. The process involves:
- Application: Submit an application to ASQ for review.
- Exam: Pass a 5.5-hour, 175-question open-book multiple-choice exam.
- Cost: The exam fee is around $538 USD. It’s often cheaper to become an ASQ member first. (Approx. ₹35,000 – ₹45,000 total).
Category 2: Regulatory Affairs
No medical device reaches a patient without clearing regulatory hurdles. In India, this means navigating the CDSCO.
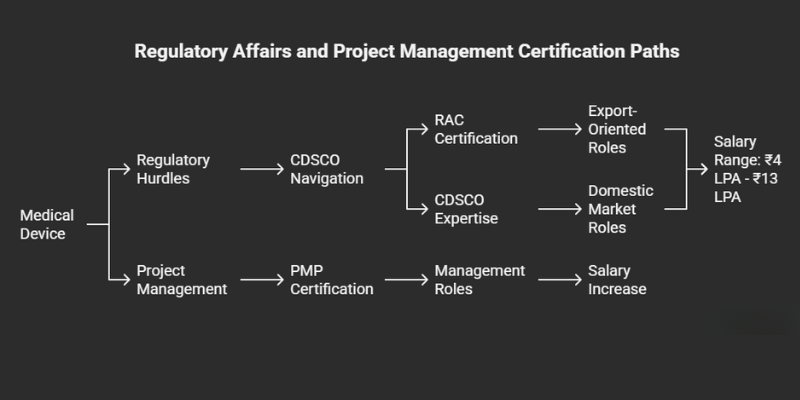
3. Regulatory Affairs Certification (RAC) & CDSCO Expertise
- What It Is: The RAC, from the Regulatory Affairs Professionals Society (RAPS), is the premier global credential [raps.org]. While there is no direct “CDSCO certification,” deep, practical knowledge of India’s Medical Device Rules (2017) is a mandatory skill for regulatory roles in India.
- Who It’s For: Mid-career BMEs, R&D engineers, and anyone looking to specialize in the path-to-market for new technologies in India or for export.
- Why It’s Valuable: A RAC is invaluable for roles in export-oriented units or MNCs. For the vast domestic market, expertise in CDSCO processes is what gets you hired. A professional with this dual knowledge can command an average salary of ₹4 LPA to ₹13 LPA [careerplanneredufair.com].
- Path to Certification:
- RAC: A bachelor’s degree and three years of regulatory/related experience are needed. The exam is challenging and covers global regulations.
- CDSCO Expertise: Gained through on-the-job experience, workshops, and short-term courses offered by various Indian industry bodies and consultancies.
- Cost (RAC): Exam fees range from $200 to $600 USD, depending on the specific exam.
Category 3: Project Management
Bringing a medical device from concept to launch is a monumental project. Certified project management skills are a game-changer.
4. Project Management Professional (PMP)®
- What It Is: The globally recognized gold standard in project management certification from the Project Management Institute (PMI) [pmi.org].
- Who It’s For: Mid-career engineers and team leads managing complex device development projects in companies like Philips, Johnson & Johnson, or Indian MedTech giants.
- Why It’s Valuable: The PMP framework is critical for navigating the long, expensive, and highly regulated medical device development cycle. It is a key credential for moving into management and leadership roles, with PMP holders in India seeing significant salary increases [netcomlearning.com].
- Path to Certification:
- Eligibility: Requires a four-year degree, 36 months of leading projects, and 35 hours of project management education.
- Exam: A 230-minute, 180-question exam.
- Cost (in India): The total investment, including training (₹15,000 – ₹40,000) and exam fees (approx. ₹23,000 for members), typically ranges from ₹40,000 to ₹70,000 [netcomlearning.com].
Category 4: Technical Specialization
For those on the front lines of equipment management in hospitals and service centers, technical certifications are essential.
5. Certified Biomedical Equipment Technician (CBET)
- What It Is: A certification from the Association for the Advancement of Medical Instrumentation (AAMI) that validates competency in managing medical equipment [aami.org].
- Who It’s For: Biomedical Equipment Technicians (BMETs), clinical engineers, and field service engineers working in hospital chains like Apollo, Fortis, or for service providers like GE and Siemens.
- Why It’s Valuable: While a US-based certification, it is a globally recognized standard for technical proficiency and patient safety. It provides foundational credibility and hands-on knowledge of the equipment lifecycle, which is highly valued in JCI-accredited hospitals in India and the Middle East.
- Path to Certification:
- Eligibility: Varies, but typically requires a combination of education (like a diploma or associate degree) and 2-4 years of full-time work experience.
- Exam: A comprehensive exam covering electronics, medical equipment function, and safety.
- Salary Impact: This certification can elevate a technician’s salary and is a stepping stone to higher-paying clinical engineering roles, which can range from ₹4 LPA to ₹12 LPA in India [careerplanneredufair.com].
Use Cases & Real-World Applications in India
Theory is one thing; application is another. Here’s how these biomedical engineer certifications translate into real-world impact in the Indian ecosystem:
- A PMP-certified R&D Manager in a Bengaluru-based MedTech park successfully leads a team to launch a new AI-powered screening tool. They use their PMP training to manage the complex timeline, mitigate CDSCO submission risks, and keep the project on budget, delivering the product to market 20% faster.
- An engineer with deep knowledge of CDSCO regulations joins a startup in Hyderabad. Her expertise allows the company to design their clinical strategy correctly from the start and prepare a flawless submission, avoiding costly delays and getting their innovative ‘Make in India’ device to patients a year ahead of schedule.
- A CQE-certified Manufacturing Engineer at a large medical implant company in Chennai uses statistical analysis to identify a flaw in a production line. By implementing a corrective action plan, she reduces the defect rate by 90%, saving the company crores in potential recalls.
- A hospital’s lead Clinical Engineer in Mumbai, who holds a CBET, develops a new predictive maintenance program for the hospital’s fleet of ventilators. His deep technical knowledge, validated by his certification, ensures equipment uptime and improves patient care in the ICU.
Pros & Cons of Pursuing Certification
Investing in a certification is a significant decision. It requires time, money, and effort. Here’s a balanced look from an Indian market perspective.
Pros (The Upside)
- Increased Earning Potential: Certified professionals earn higher salaries, with potential for significant jumps into higher income brackets in India [careerplanneredufair.com].
- Global & Local Opportunities: Global certifications (PMP, CQE) open doors to MNCs in India and abroad, while local expertise (CDSCO) is key for domestic roles.
- Improved Job Security: Specialized, validated skills make you a more valuable asset in a competitive job market.
- Expanded Professional Network: Joining bodies like PMI, RAPS, and ASQ connects you with a global community of experts.
- Demonstrated Commitment: It shows employers you are proactive, dedicated, and passionate about your field.
Cons (The Considerations)
- Significant Cost: Training and exam fees can be a substantial investment, often running into lakhs of rupees.
- Time Commitment: Preparing for exams requires hundreds of hours of study, which can be challenging to balance with a full-time job.
- Maintenance Requirements: Most certifications require continuing education and renewal fees to remain active.
- No Guarantee of Success: Passing exams is not guaranteed and may require multiple attempts, adding to the cost.
- Experience is Still Key: A certification complements experience; it does not replace it. You cannot certify your way out of needing hands-on skills.
Common Questions & FAQs
I’m a B.Tech student in India. Should I get certified before I graduate?
For most certifications like PMP or RAC, you need professional experience. However, you can start preparing. Consider the Certified Associate in Project Management (CAPM)® from PMI as a precursor to the PMP. Your main focus as a student should be on gaining practical experience through internships at Indian MedTech companies and research projects at institutions like IITs or IISc.
Is a certification better than an M.Tech?
They serve different purposes. An M.Tech provides deep academic knowledge, often required for R&D or academic roles. A certification validates specific, industry-standard skills (e.g., quality control, regulatory compliance). For the best career growth in India, an M.Tech from a reputed university combined with a strategic professional certification is a powerful combination.
Are these international certifications recognized in India and neighboring countries?
Absolutely. Global certifications like PMP, CQE, and ISO 13485 Lead Auditor are highly respected, especially by multinational corporations operating in India, Bangladesh, Sri Lanka, etc., and by domestic companies that export their products. They demonstrate that your skills meet a global standard. However, for roles focused purely on the domestic market, they are most powerful when paired with a deep understanding of local regulations like those from the CDSCO.
Will my employer in India pay for my certification?
Many large Indian companies and MNCs have professional development budgets and will reimburse you for costs, especially if the certification directly benefits your role. It’s always worth discussing with your manager. Frame it as an investment that will bring valuable skills back to the company.
Conclusion: Your Next Step to a More Rewarding Career in South Asia
The landscape of biomedical engineering in 2026 and beyond will be defined by rapid innovation and increasing complexity, with India and its neighbors at the heart of much of this growth. A degree provides the foundation, but continuous learning through certification is what will build your career. The biomedical engineer certifications and skills we’ve covered—PMP, CDSCO expertise, CQE, ISO 13485, and CBET—are more than just acronyms. They are strategic investments that can lead to higher salaries, global opportunities, and more fulfilling work right here in the region.
My advice is to be intentional. Don’t just collect certifications. Identify where you want to go in your career—be it in a bustling Indian metro or an emerging market in a neighboring country—and choose the credential that builds the most direct bridge to get you there. Start planning today, and commit to the journey. The rewards are well worth the effort.
Ready to Bridge Innovation and Your Career?
Have questions about which certification is right for you? Or do you have experience with a certification you’d like to share? Leave a comment below! Let’s empower each other with shared knowledge.
And if you’re looking for expert guidance on your MedTech projects or career path, explore my consultation services.Explore My Services
