Introduction: A Precise Weapon Against Cancer
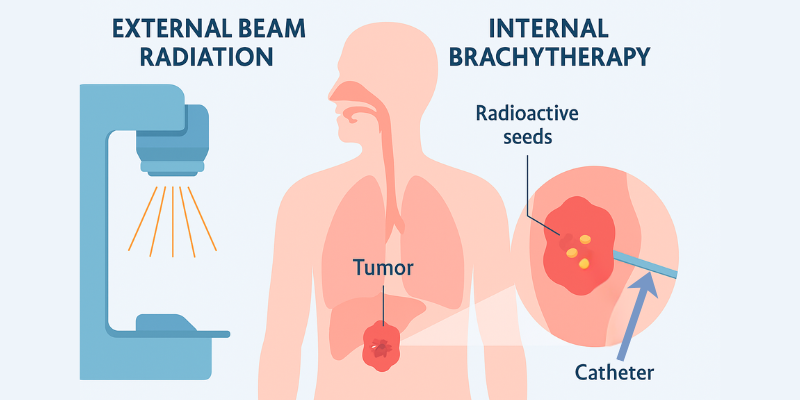
In the landscape of modern oncology, precision is paramount. Brachytherapy, a form of internal radiation therapy, stands out as a powerful example of targeted cancer treatment. Unlike conventional external beam radiation, brachytherapy works from the inside out, placing a radioactive source directly within or next to a tumor. This method delivers a high, localized dose of radiation to destroy cancer cells while minimizing damage to surrounding healthy tissue.
At the heart of this sophisticated treatment is the brachytherapy machine. These devices, ranging from complex, computer-controlled afterloaders to systems for planning permanent seed implants, are the engines that drive this precise therapy. This article provides a comprehensive overview of brachytherapy machines, exploring their types, costs, technical specifications, and patient implications, offering valuable insights for medical professionals, patients, researchers, and healthcare investors alike.
Table of Contents
What is Brachytherapy and How Does it Work?
Brachytherapy, derived from the Greek word “brachy” meaning “short distance,” is a type of radiotherapy where radioactive material is placed inside the body. This material, often in the form of seeds, ribbons, or capsules, emits radiation that damages the DNA of cancer cells, preventing them from dividing and growing. This internal delivery is its key advantage.
Brachytherapy allows a healthcare team to use higher doses of radiation than would be possible with external radiation. This is because it delivers radiation directly to the treatment area, lowering the risk of harming nearby healthy tissue. As the Mayo Clinic notes, this can also lead to shorter overall treatment times.
Unlike External Beam Radiation Therapy (EBRT), where radiation beams are directed from outside the body, brachytherapy machine delivers radiation directly at the tumor site. This targeted approach ensures a highly conformal dose, precisely matching the tumor’s shape and significantly protecting nearby healthy organs.
Types of Brachytherapy Machines and Delivery Systems
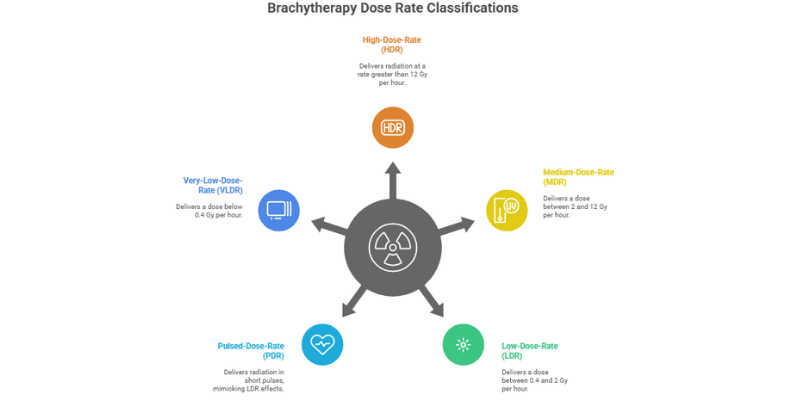
The term “brachytherapy machine”; encompasses a range of technologies, primarily differentiated by the rate at which the radiation dose is delivered. This dose rate is a critical factor that influences the biological effect of the treatment and the clinical workflow.
Dose Rate Classifications: The Core Difference
The International Commission on Radiation Units and Measurements (ICRU) categorizes brachytherapy based on dose rate. These classifications are fundamental to understanding the different types of brachytherapy machines and their applications:
- High-Dose-Rate (HDR): Delivers radiation at a rate greater than 12 Gray (Gy) per hour.
- Medium-Dose-Rate (MDR): Delivers a dose between 2 and 12 Gy per hour. This range is less commonly used today.
- Low-Dose-Rate (LDR): Delivers a dose between 0.4 and 2 Gy per hour.
- Pulsed-Dose-Rate (PDR): A hybrid approach that uses an HDR source to deliver radiation in short pulses, typically once per hour, mimicking the radiobiological effects of LDR.
- Very-Low-Dose-Rate (VLDR/ULDR): Delivers a dose below 0.4 Gy per hour, characteristic of permanent seed implants.
Increasing the dose rate enhances the radiation’s biological impact, but it must be precisely managed to protect healthy tissue. As studies note, HDR brachytherapy machines use fractionated sessions—short, controlled doses that allow normal cells to repair safely between treatments.
High-Dose-Rate (HDR) Afterloaders
Modern HDR brachytherapy machines use advanced, computer-controlled remote afterloaders developed by leading manufacturers like Varian and Elekta. These systems contain a highly radioactive source—usually Iridium-192—that is precisely guided through non-radioactive applicators such as catheters or needles. The source dwells at programmed positions for calculated durations, ensuring an accurate and effective radiation dose directly to the tumor site.
Key Features & Advantages:
- Radiation Safety: Healthcare staff are not exposed to radiation, as the source is only deployed after everyone has left the shielded treatment room.
- Speed and Convenience: Treatment sessions are short, often lasting only minutes, and can be performed on an outpatient basis. A typical HDR course may involve several sessions over a few days or weeks.
- Dose Optimization: The computer-controlled movement of the source allows for highly optimized and sculpted radiation doses.
Low-Dose-Rate (LDR) Systems
LDR brachytherapy is the oldest form of this treatment and has a long history of clinical success, particularly for prostate cancer. Unlike HDR, LDR typically involves the permanent implantation of tiny radioactive “seeds” (e.g., Iodine-125 or Palladium-103) directly into the tumor. These seeds emit a low dose of radiation over several weeks or months until their radioactivity decays.
The “machine” aspect of LDR is less about a single delivery unit and more about the integrated system of planning and implantation. This includes:
- Treatment Planning Software: Systems like Varian’s VariSeed are used to determine the optimal number and placement of seeds.
- Implantation Tools: Specialized needles and templates are used to precisely place the seeds according to the plan, often under ultrasound guidance.
Temporary LDR implants also exist, where applicators are left in place for several days to deliver the dose. Studies have shown very high rates of cancer control with LDR for prostate cancer, making it a durable and effective option.
Emerging Technology: Electronic Brachytherapy (EB)
A significant innovation is electronic brachytherapy (EB), which uses a miniaturized, low-energy X-ray source instead of a radionuclide. Systems like the Xoft Axxent and Zeiss Intrabeam generate radiation only when powered on.
Key Advantages:
- No Radioactive Material: Eliminates the need for handling, storing, and disposing of radioactive sources.
- Reduced Shielding: The low-energy X-rays require significantly less room shielding than traditional HDR sources.
- Safety: The device is not radioactive when turned off, enhancing safety for staff and patients.
As described in a review on the topic, the rapid dose fall-off of these low-energy sources is a highly desirable property, further reducing the dose to surrounding normal tissues.
Scientific/Technical Aspects: A Deeper Dive
The Radiobiology of Dose Rates
The choice between HDR and LDR is not just a matter of convenience; it has profound radiobiological implications. The biological effect of radiation is governed by the “4 R’s of Radiobiology”: Repair of sublethal damage, Reoxygenation of hypoxic tumor cells, Redistribution of cells into sensitive phases of the cell cycle, and Repopulation of cells.
LDR treatment, delivered continuously over days, allows for significant repair in healthy tissues while continuously damaging cancer cells, which may have impaired repair mechanisms. HDR, delivered in a short, intense burst, allows for less repair during the fraction. To make different treatment schedules comparable, radiobiologists use mathematical models like the Linear-Quadratic (LQ) model. This model helps calculate the Biologically Effective Dose (BED) and convert doses between different fractionation schemes (e.g., calculating an HDR dose that is equivalent to a proven LDR regimen), often expressed as EQD2 (the equivalent dose in 2 Gy fractions). The GEC-ESTRO Handbook provides a detailed explanation of these concepts, noting their importance for comparing treatment effects.
Treatment Planning Systems (TPS)
Modern brachytherapy is impossible without advanced Treatment Planning Systems (TPS). These software platforms, such as Varian’s BrachyVision, are the “brains” behind the brachytherapy machine. They integrate patient imaging (CT or MRI) to create a 3D model of the tumor and surrounding organs. Physicists and oncologists then use the TPS to:
- Digitally reconstruct the position of applicators or needles.
- Define the target volume and organs at risk.
- Optimize the radiation dose distribution by controlling source dwell times and positions (for HDR) or seed placement (for LDR).
- Calculate the final dose using established algorithms like the AAPM TG-43 formalism.
The goal is to deliver a lethal dose to the tumor while keeping the dose to healthy tissues below tolerance limits.
Quality Assurance and Safety Standards
The precision of a brachytherapy machine demands rigorous quality assurance (QA). Professional bodies like the American College of Radiology (ACR) and the American Association of Physicists in Medicine (AAPM) have established technical standards for both HDR and LDR brachytherapy. These standards cover:
- Equipment Performance: Regular checks of the source strength, positional accuracy of the afterloader, and timer functionality.
- Treatment Planning Verification: Independent checks of dose calculations.
- Radiation Safety: Procedures to minimize radiation exposure to staff and the public, overseen by regulatory bodies like the U.S. Nuclear Regulatory Commission (NRC).
- Personnel Qualifications: Strict requirements for the training and expertise of medical physicists, dosimetrists, radiation therapists, and physicians.
These comprehensive QA programs are essential to ensure that every treatment is delivered safely and accurately.
Hospitals seeking to integrate a high-dose‐rate brachytherapy machine should assess not only the device cost, but also room shielding requirements, workflow, staffing and regulatory licensing. For a full breakdown of how to plan, budget and equip a modern oncology center, refer to our comprehensive oncology center setup guide.
Use Cases & Real-World Applications
Brachytherapy is a versatile treatment used for many types of cancer, often where a highly localized dose is advantageous. Common applications include prostate, cervical, breast, skin, and esophageal cancers. It is most frequently used for prostate and cervical cancers.
Prostate Cancer: A Tale of Two Approaches (LDR vs. HDR)
Prostate cancer is one of the most common indications for brachytherapy. Both LDR and HDR are widely used, and the choice often depends on the cancer’s risk profile, patient anatomy, and physician/patient preference.
- LDR Brachytherapy: Often called “seed implantation,” this involves a one-time procedure where dozens of tiny radioactive seeds are permanently placed in the prostate. It is a well-established monotherapy for low- and favorable intermediate-risk prostate cancer.
- HDR Brachytherapy: This involves the temporary placement of catheters into the prostate, through which a high-activity source is delivered in one or more fractions. It can be used as a monotherapy or as a “boost” in combination with external beam radiation for higher-risk disease.
A 2019 report noted that there is no direct clinical evidence supporting the superiority of one method over the other regarding tumor control or toxicity, making patient-centered decision-making crucial.
Gynecological and Breast Cancers
HDR brachytherapy is a cornerstone in the curative treatment of cervical cancer, typically used as a boost after external beam radiation. For breast cancer, brachytherapy is a key component of Accelerated Partial Breast Irradiation (APBI), a shorter course of radiation for early-stage patients following a lumpectomy. This targeted approach treats only the area around the tumor bed, sparing the rest of the breast and nearby organs like the heart and lungs.
The Patient Experience: What to Expect
For patients, understanding the process can alleviate anxiety. While specifics vary, the general steps for temporary (HDR/LDR) brachytherapy are:
- Implant Placement: Applicators (catheters or needles) are placed in or near the tumor, often under anesthesia.
- Simulation & Planning: Imaging (CT/MRI) is performed with the applicators in place to create a precise treatment plan.
- Treatment Delivery: The patient is taken to a shielded room where the brachytherapy machine is connected to the applicators to deliver the radiation. This part is painless.
- Implant Removal: Once the treatment course is complete (after minutes for HDR, or days for temporary LDR), the applicators are removed.
For permanent LDR seed implants, the procedure is a single session. Afterward, patients may need to take temporary precautions, such as limiting close contact with pregnant women and small children, as the seeds emit low levels of radiation that decay over time. Most side effects are manageable and depend on the treated area.
Market Trends & Investment Insights
The brachytherapy market is characterized by steady growth, driven by the rising global incidence of cancer and continuous technological innovation. For hospital administrators and investors, understanding these dynamics is key to making informed decisions.
Market Size and Growth
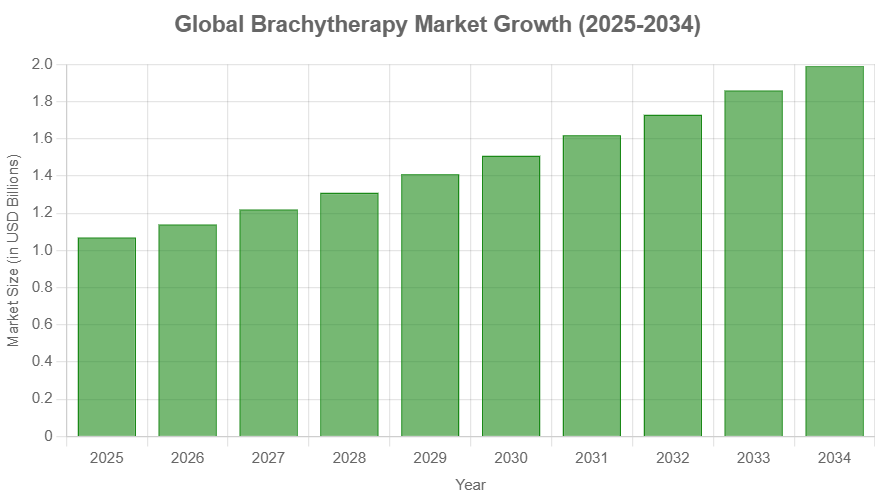
The global brachytherapy market is on a significant upward trajectory. According to Precedence Research, the market was valued at approximately $0.99 billion in 2024 and is projected to reach $1.99 billion by 2034, growing at a compound annual growth rate (CAGR) of 7.23%. Prostate cancer applications currently hold the largest market share, though breast cancer applications are projected to grow at the fastest rate. North America represents the largest regional market, but Asia Pacific is expected to see the most rapid growth.
Cost Analysis: Equipment and Procedures
The investment in a brachytherapy program is substantial. The cost of a brachytherapy machine, particularly an HDR afterloader, can range from several thousand to several hundred thousand dollars, depending on the manufacturer (e.g., Varian, Elekta) and its features. Used or refurbished equipment is also available at a lower price point.
From a patient or payer perspective, the cost of a brachytherapy procedure can vary widely. Reports suggest a range of $1,200 to $4,800 per session, depending on the treatment type, complexity, and geographic location. To optimize costs and efficiency, healthcare institutions are increasingly using methodologies like Time-Driven Activity-Based Costing (TDABC) to analyze and streamline brachytherapy workflows. This approach helps identify inefficiencies and informs resource allocation.
Key Market Drivers and Future Outlook
The market’s growth is fueled by several factors:
- Rising Cancer Prevalence: An aging global population and lifestyle factors are contributing to a higher incidence of cancer.
- Technological Advancements: Innovations are making brachytherapy more precise, safer, and applicable to more cancer types.
- Patient Preference: The convenience of shorter treatment courses (like APBI) is an attractive option for many patients.
The future of brachytherapy is bright, with research focused on integrating cutting-edge technologies. Artificial intelligence (AI) is poised to revolutionize treatment planning by automating tasks like applicator reconstruction and organ segmentation. Other promising areas include robotic-assisted delivery for enhanced precision and the use of 3D-printed custom applicators for personalized treatments. These innovations promise to solidify brachytherapy’s role as a cornerstone of localized cancer care.
Pros & Cons of Brachytherapy
Advantages
- High Precision: Delivers a maximum radiation dose directly to the tumor.
- Tissue Sparing: Minimizes radiation exposure to surrounding healthy organs and tissues.
- Shorter Treatment Times: Overall treatment can often be completed in less time than traditional external radiation.
- Reduced Side Effects: The localized nature of the treatment can lead to fewer systemic side effects.
Disadvantages and Side Effects
- Invasive Nature: Requires the placement of applicators or seeds into the body.
- Limited Applicability: Best suited for localized, well-defined tumors and not for metastatic disease.
- Potential Side Effects: Risks are specific to the treatment area. For example, prostate brachytherapy can cause urinary symptoms or erectile dysfunction. These side effects can be long-term.
- Requires Expertise: Demands a highly skilled, multidisciplinary team and specialized facilities.
Common Questions & FAQs
Is brachytherapy painful?
The placement of applicators is done under anesthesia to prevent pain. Some discomfort or soreness may be felt at the site after the procedure, but this is typically managed with pain medication. Pain medicine is provided to ease any discomfort.
Will I be radioactive after treatment?
If you have temporary brachytherapy (like HDR), the radioactive source is removed completely. You will not be radioactive and are not a danger to others. If you have permanent brachytherapy (like LDR seeds), the implants emit a low level of radiation that decays over time. You may be advised to take simple, temporary precautions. The risk to others is usually low.
Can brachytherapy be combined with other treatments?
Yes. Brachytherapy is often used in combination with other treatments like external beam radiation therapy (EBRT), surgery, or chemotherapy to improve outcomes. This multimodal approach is common.
What are the main differences between HDR and LDR brachytherapy?
The primary difference is the dose rate and treatment duration. HDR uses a high-intensity source for a few minutes per session over several treatments. LDR uses a low-intensity source delivered continuously over many days (temporary) or months (permanent seeds).
Conclusion: The Enduring and Evolving Role of Brachytherapy
From its origins over a century ago to today’s AI-driven planning systems, brachytherapy has remained a vital and evolving modality in cancer treatment. The modern brachytherapy machine is a testament to technological progress, enabling oncologists to deliver highly precise, potent, and personalized radiation therapy. Its ability to maximize tumor destruction while minimizing collateral damage ensures its place as a cornerstone of curative cancer care.
As technology continues to advance, brachytherapy will become even more refined, accessible, and effective. For healthcare providers considering an investment in advanced oncology solutions, or for patients exploring their treatment options, understanding the nuances of the modern brachytherapy machine is the first step toward leveraging this powerful technology.
